Abstract
The relationship between soy intake (SI) and breast cancer (BC) has been widely investigated with limited information on the significance of hormone receptor status of BC on the association. This study assessed the relationship between SI and BC risk in the context of oestrogen receptor (ER) status of BC. We meta-analyzed data from published studies on SI and BC after a methodical search of EMBASE, PubMed and Cochrane Library through December 2019. Summary estimates with 95% confidence intervals (CI) were presented using a random-effects model. Eighteen (5 cohorts and 13 case-control) studies, were included in this meta-analysis and SI was inversely associated with BC risk [OR (95%) for highest vs. lowest soy food intake = 0.88 (0.84–0.92), P < 0.001, I2 = 76.1%, Egger's p-value = 0.425] among all women. The inverse relationship was stronger among premenopausal women [OR (95%) = 0.79 (0.71–0.87), P < 0.001, I2 = 77.3%, Egger's p-value = 0.644]. In addition, SI was inversely associated with BC risk among ER-negative (–) BC women [OR (95%) = 0.71 (0.57–0.90), P = 0.013, I2 = 72.0%, Egger's p-value = 0.355] and among ER-positive (+) BC women [OR (95%) = 0.87 (0.79–0.96), P = 0.008 I2 = 74.6%, Egger's p-value = 0.061]. SI appears inversely associated with BC risk, with a stronger inverse association among pre-menopausal and ER-negative BC women.
Keywords: Food science, Epidemiology, Cancer research, Nutrition, Soy, Breast cancer, Oestrogen receptor, Meta-analysis
Food science; Epidemiology; Cancer research; Nutrition; Soy; Breast cancer; Oestrogen receptor; Meta-analysis
1. Introduction
Breast cancer (BC) is a predominant form of cancer among women [1], with a higher incidence among women from the Western population [2]. It is a leading cause of death among women in China [3], with over 1.6 million cases and 1.2 million BC-related annual deaths [4]. Also, Soy is a customary foodstuff among Asians [5] and has been extensively associated with BC risk. Several population-based studies have reported the relationship between higher dietary soy intake (SI) and BC risk; some found an inverse relationship [6, 7], some an aggressive relationship [8] and others no relationship [9].
Manipulating the metabolism and impact of sex hormones on mammary tissues has been an effective stratagem for BC management. For example, blocking estrogen action/production has been widely used as adjuvant therapy in BC treatment [10]. However, consensus on the significance of dietary SI in BC risk, taking into account BC hormone receptor status (HRS) is lacking.
Shu et al [10] reported the potential effect of SI on BC recurrence and mortality differed by HRS of BC tumours. In that study, dietary SI was inversely associated with BC risk among women with estrogen receptor-positive (ER+) BC tumour but not among those with estrogen receptor-negative (ER–) BC tumours. These findings were similar to reports from Kang et al [11] and Zhang et al [12]. Contrariwise, Zhang et al [13] alongside recently published findings by Baglia et al [7] reported the SI-BC link is independent of HRS.
These disparities in the conclusion of previously published studies (likely as a result of choice of population, sample size, magnitude and time of exposure to SI as well as study designs among others) promotes complicity in drawing lucid conclusions on whether HRS (particularly oestrogen) of BC phenotypes modifies SI-BC link. Furthermore, despite several systematic reviews and meta-analysis [14, 15, 16, 17, 18, 19, 20] on this subject, none has been able to summarize evidence on the significance of HRS on the SI-BC relationship.
In the light of these gaps, we attempted to explore the relationship of SI and BC risk using a meta-analysis of previously published studies stratified by ER status of BC to offer substantial evidence that informs well-articulated public health advisory on dietary SI.
2. Materials and methods
2.1. Search strategy
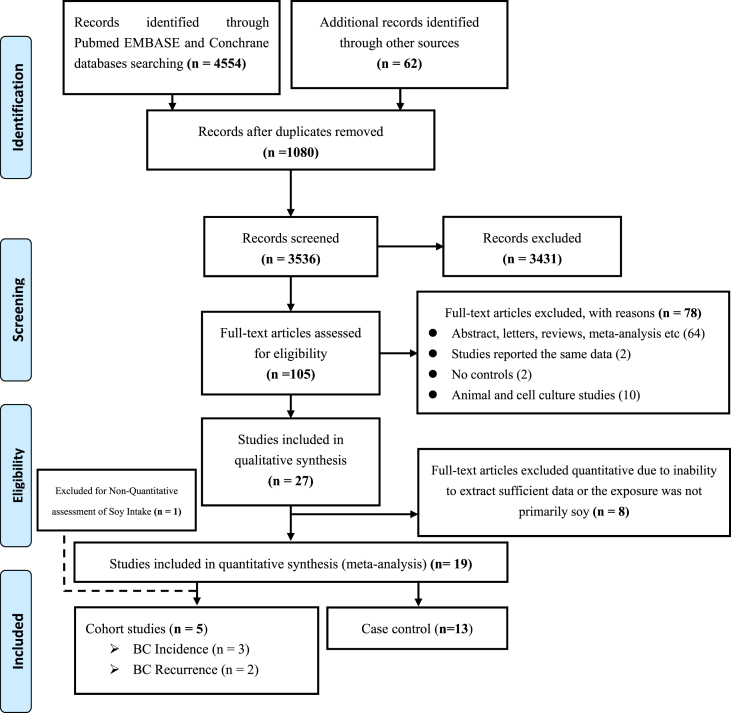
First, our meta-analysis was prospectively catalogued in PROSPERO electronic records (CRD42017065249) and conducted using MOOSE guidelines [21]. To identify previously published studies for the meta-analysis, two members of the review team independently searched electronic databases; Cochrane Library, PubMed and EMBASE through December 2019. The search was conducted without restriction using a permutation of the following structured terms alone or in combination; “breast cancer” or “breast tumour” and “soy” or “soy intake” or “tofu” or “soy food” or “soybean” or “isoflavones”. Also, a manual search of the reference list and supplementary files of retrieved articles was carried out for additional data. The principle, method and report of the literature search strategy for the meta-analysis are presented in Figure 1.
Figure 1.
PRISMA flowchart study selection in the meta-analysis.
Articles were included in the meta-analysis after meeting the following pre-defined criteria: (a) the morbidity endpoint of significance was BC among women; (b) the exposure of interest was the consumption of soy foods/protein or soy-based foods evaluated ≥12 months before diagnosis; (c) reported risk estimates (95%CI) with multivariate adjustment for confounding variables; and (d) reported data to compute these estimates [e.g., the incidence of BC or BCR (breast cancer re-occurrence) in the same study or differently]. Two reviewers autonomously appraised titles and abstracts of retrieved articles about the eligibility for inclusion. Full-length articles only were considered, but abstracts, in-vitro studies, animal studies, case reports, reviews, articles with unpublished information and/or language of publication is not the English language were excluded. Also, any contrast in the assessment was harmonized either by consensus or by recourse to a third reviewer. Where data overlap was observed among published studies, the study with detailed information was included. Where eligibility of article(s) for inclusion was uncertain among reviewers, a full-text copy was obtained and resolved accordingly.
2.2. Data extraction
Authors' name, publication year, geographical region, study name, study population, the measure of exposure, disease endpoint, incidence BC/BCR, BC/BCR cases, ascertainment and range of exposure were collected using a predefined data collection form. Data extraction was implemented independently by two reviewers, and inconsistencies were resolved upon repeated examination of the studies or/and in recourse to a third reviewer.
2.3. Quality evaluation of studies
Using the Newcastle-Ottawa Scale (NOS) checklist [22], we evaluated the quality of observational studies included in our meta-analysis and risk of bias in the light of the following parameters; selection, comparability and exposure/outcome.
2.4. Statistical analysis
Statistical analyses were carried out using STATA 12.1, StataCorp USA. Using the Mantel-Haenszel method, we meta-analyzed data on cases/incidence and population in the highest compare to the lowest quantiles of soy intake exposure using the random effect model (REM) which takes into cognizance between- and within study variations, to obtain more conservative estimates compared to the fixed effect model. Also, subgroup analyses according to estrogen receptor BC phenotypes, menopausal status and population differences of women in this study were explored to test the effect of these variations on the overall finding of this meta-analysis. The summary measures were presented as forest plots where the size of data markers (squares) corresponds to the inverse of the variance of the natural logarithm of risk difference from each study, and the diamond indicates odds ratio.
Heterogeneity of the overall estimates and the extent of variability across studies were computed using I2-test statistics, and where substantial heterogeneity (i.e. I2 exceeded 50% or P < 0.05) was observed, a REM was applied to obtain effect estimates; else a fixed-effect model (FEM) was considered. The FEM postulate that observed risk differences were an after-effect of chance. REM assumes relative risk (RR)/odds ratio (OR) in the different studies is not similar but follows a symmetrical distribution. Probabilities were two-tailed and P < 0.05 was considered statistically significant. The area of the black square of the forest plots connotes the weighted contribution of each study. Sensitivity analysis and publication bias of the results were evaluated using the leave-one-out method and Begg's funnel plots (statistically assessed using Egger's regression intercept test) respectively.
3. Results
3.1. Literature search
The PRISMA flowchart summarizing the process of the study selection is described in Figure 1. We identified an initial record of 4,616 from the primary literature search and 1,080 duplicates records were excluded. After examining titles and abstracts, 3,536 records were excluded and 105 studies were included for full-text assessment. Seventy-eight studies that did not meet the inclusion criteria were excluded, and a total of 27 studies met the inclusion criteria for the qualitative data mining. Furthermore, 7 studies [9, 23, 24, 25, 26, 27, 28] were excluded in the meta-analysis given the lack of sufficient information for the quantitative review. In all, 18 studies involving 5 cohort studies (BC incidence [6, 7, 29], BC recurrence [10, 30]) and 13 case-control studies [31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43] on SI and BC were included in the meta-analysis after excluding 8 studies where exposure to soy-based foods was not clearly defined.
3.2. Characteristics of the included studies
Characteristics of studies included in the meta-analysis are summarized in Tables S1 and S2. Studies included were conducted in the following geographical locations; the USA, Germany, China, Singapore, Korea and Japan and published between 1996 and 2017.
Also, methodological quality evaluation of studies included in the meta-analysis is presented in SM Table S4. Low risk of bias was observed among most of the studies included in the meta-analysis.
3.3. Soy intake and risk of BC
Higher SI was associated with reduced risk of BC in all studies OR (95%CI): 0.88 (0.84–0.92), P < 0.001, I2 = 76.1%, Egger's p-value = 0.108 (Table 1 and Figure S1). The relationship was attenuated among cohort studies; RR (95%CI): 0.96 (0.84–1.09), P = 0.511, I2 = 72.5%, Egger's P-value = 0.838 but remained among case-control studies; OR (95%CI): 0.86 (0.82–0.91), P < 0.001, I2 = 77.9%, Egger's P-value = 0.445.
Table 1.
Overall estimates, 95% CI, I2 and Egger's P-value in the meta-analysis.
| N | Effect estimate (95% CI) | P-value | I2 (%) | Egger's P-value | |
|---|---|---|---|---|---|
| All studies‡ | 18 | 0.88 (0.84–0.92) | <0.001 | 76.11 | 0.425 |
| RR for Cohort studies only | 5 | 0.96 (0.84–1.09) | 0.511 | 72.50 | 0.838 |
| OR for Case control studies only | 13 | 0.86 (0.82–0.91) | <0.001 | 77.98 | 0.445 |
N = number of studies.
RR – relative risk.
OR – odds ratio.
Includes both cohort and case-control studies.
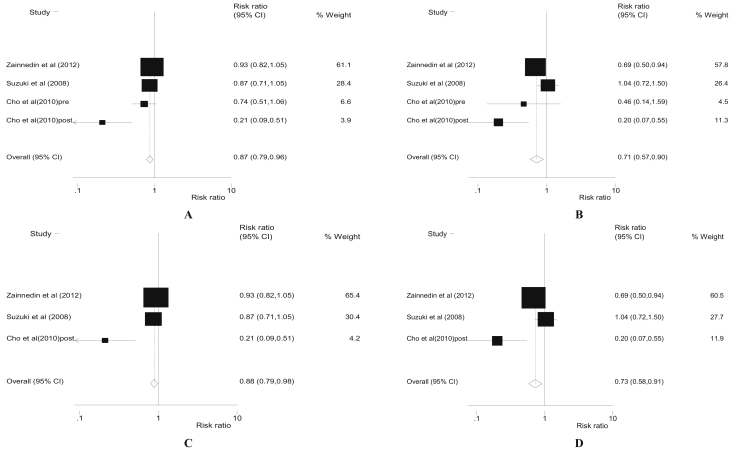
Also, stratifying the meta-analysis by HRS of BC tumors among women (Table 2 and Figure 2), we found higher SI was associated with reduced risk of BC; OR (95%CI): 0.87 (0.79–0.96), P = 0.008, I2 = 74.6%, and Egger's P-value = 0.061 among women with BC tumors sensitivity to oestrogen hormone (ER+). Similarly, odds of BC for higher SI was; OR (95%CI): 0.88 (0.79–0.98), P = 0.016, I2 = 81.5%, Egger's p-value = 0.083 among ER + post-menopausal women.
Table 2.
Odds ratio of subgroup analysis by estrogen receptor status in the meta-analysis.
| N | RR/OR (95% CI) | P-value | I2 (%) | Egger's P-value | |
|---|---|---|---|---|---|
| ER + | |||||
| All studies† | 4 | 0.87 (0.79–0.96) | 0.008 | 74.64 | 0.061 |
| Pre-menopausal† | 1 | 0.65 (0.32–1.32)∗ | 0.120∗ | ||
| Post- menopausal† | 3 | 0.88 (0.79–0.98) | 0.016 | 81.58 | 0.083 |
| ER– | |||||
| All studies† | 4 | 0.71 (0.57–0.90) | 0.013 | 72.04 | 0.355 |
| Pre-menopausal† | 1 | 0.78 (0.14–4.44)∗ | 0.611∗ | ||
| Post- menopausal† | 3 | 0.73 (0.58–0.91) | 0.006 | 80.25 | 0.501 |
N = number of studies.
Case-control studies only.
Data from individual study.
Figure 2.
Forest plots of for higher dietary soy intake and risk of breast cancer for all ER + women (A), all ER- women only (B), ER + postmenopausal women only (C) and ER- postmenopausal women (D).
On the other hand, the SI-BC risk relationship was stronger; OR (95%CI): 0.71 (0.57–0.90), P = 0.013, I2 = 72.0%, Egger's p-value = 0.355 among women with ER– BC tumors sensitivities. Similarly, the OR (95%CI) of BC risk among ER– post-menopausal women was 0.73 (0.58–0.91), P = 0.006, I2 = 80.2%, Egger's P-value = 0.501. Also, higher SI-BC link was significantly protective (though the relationship appears stronger among premenopausal women) independent of menopausal status (Table 3).
Table 3.
Subgroup analysis by menopausal status in the meta-analysis.
| N | RR/OR (95% CI) | P-value | I2 (%) | Egger's P-value | |
|---|---|---|---|---|---|
| Premenopausal | |||||
| All studies‡ | 9 | 0.79 (0.71–0.87) | <0.001 | 77.31 | 0.644 |
| RR for Cohort studies only | 3 | 0.50 (0.35–0.70) | <0.001 | 53.27 | 0.258 |
| OR for Case control studies only | 6 | 0.85 (0.76–0.94) | <0.001 | 77.99 | 0.559 |
| Postmenopausal | |||||
| All studies‡ | 11 | 0.88 (0.83–0.94) | <0.001 | 91.95 | 0.047 |
| RR for Cohort studies only | 3 | 0.94 (0.78–1.14) | 0.546 | 58.50 | 0.095 |
| OR for Case control studies only | 8 | 0.87 (0.81–0.93) | <0.001 | 64.32 | 0.119 |
N = number of studies.
RR – relative risk.
OR – odds ratio.
Includes both cohort and case-control studies.
Furthermore, higher SI exposure was inversely associated with reduced risk of BC independent of the ethnic setting (Table S5); Asian populations OR (95%CI): 0.88 (0.82–0.94), P < 0.001, I2 = 79.5%, Egger's P-value = 0.243 and Western populations; OR (95%CI): 0.88 (0.85–0.93), P = < 0.001, I2 = 58.3%, Egger's P-value = 0.186. Similarly, stratification of our meta-analysis by QoS revealed SI was associated with BC risk (though slightly attenuated) independent of the QoS.
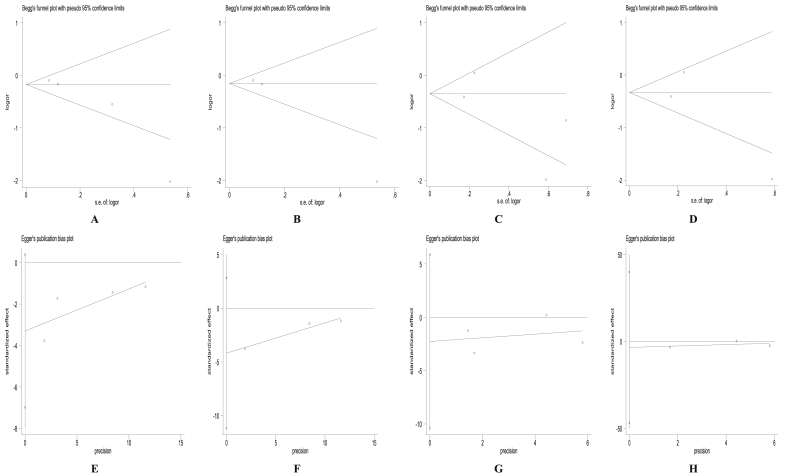
3.4. Publication bias
Begg's funnel plots and Egger regression test (Figure 3 and Figure S2) suggested no significant evidence of publication bias for the studies included in the meta-analysis.
Figure 3.
Begg's plots for higher dietary soy intake and risk of breast cancer for all ER + women (A), ER + postmenopausal women only (B), all ER- women only (C), ER- postmenopausal women (D) and Egger's regression intercept test for higher dietary soy intake and risk of breast cancer for all ER + women (E), ER + postmenopausal women only (F), all ER- women only (G), ER- postmenopausal women (H).
3.5. Sensitivity analysis
The strength of individual study on the overall results of the meta-analysis was evaluated by excluding individual study at a time to test the presence of significant alterations in the overall result of the meta-analysis (Table S6). In brief, few studies exerted profound but insignificant weight on the overall results of the meta-analysis.
4. Discussion
The SI-BC link has been explored by some reviews and meta-analysis [14, 15, 17, 19, 20, 44, 45, 46, 47] and reports [7, 20, 29, 41] with a limited conclusion on the significance of HRS. Our report being the first revealed higher SI is not only inversely with BC risk, but also the protective relationship is stronger among ER– BC and premenopausal women. As against previous meta-analyses (Table S3), our meta-analysis presents data on the significance of HRS in the relationship between SI and BC risk.
Soy is a healthy, exceptional and inexpensive source of functional nutrients such as protein, fibre and saponin [48]. Isoflavone from soy has been widely reported in epidemiological studies to be primarily responsible for the protective SI-BC link [47]. Isoflavone is not singly consumed in the diet and as well perhaps not the only functional compound reported to improving BC risk in soy. For example, soy is the sole vegetable source with complete protein alongside high and low polyunsaturated and saturated fats content respectively [48].
In addition, Lima et al [49] have reported the inhibitory potential of soy protein extract on cellular matrix metalloproteases (MMP-9). MMP-9 are enzymes (from Zn-dependent neutral endopeptidases) expressed in tumour invasion and metastasis. Soy-protein also likely exerts inhibitory potential on BC progression and metastasis by acting as MMP-9 inhibitors thereby reducing BC risk. Yan et al [50], also demonstrated the significant potential of soy protein extract intakes in alleviating metastasis of BC in mice models. Similarly, Farvid et al [51] recently reported decreased BC risk (due to higher fibre intakes) in a cohort of premenopausal women after 20 years of follow-up. In the same vein, a recent meta-analysis reported fibre consumption was significantly associated with a reduced BC risk, particularly among postmenopausal women [52]. Also, soy saponins have been suggested via multiple mechanisms to be involved in the anti-cancer effect of soy [53].
Moreover, the anti-carcinogenic effects of isoflavone (from soy) on BC in culture medium [54, 55, 56], animal model [57] and some human trials [7, 24, 32] is worth mentioning but also subject to debate because of its anti-estrogenic effects [58]. On one hand, isoflavones from soy potentially inhibit tumorigenesis by blocking the enzymes crucial for DNA replication, signal transduction and metastasis [59, 60], suppress the invasiveness of BC cell (through series of interlinked cellular processes) by inhibiting the transcription of NF-kappaB/AP-1-dependent and -independent pathways to promote the down-regulation of urokinase-type plasminogen activator in the BC cell MDA–MB–231 [54]. On the other hand, isoflavone can bind to oestrogen receptors (given it is stereo-chemically associated with oestrogen) and thus potentially compete for the same receptor binding with oestrogen – a BC sensitive hormone. At elevated oestrogen levels, oestrogen affinity increases endogenously and isoflavone effect on BC is weakened thus alleviating BC risk may be more or less weakened [55]. This may account for why some trials [61, 62] reported a null association of isoflavone-BC link.
Our finding that the SI-BC risk appears stronger among ER– BC is in tandem with the suggestion of Stubert and Gerber [63] (largely attributable to the unreliability of oestrogenic behaviour of isoflavone (a rich component of soy) and a report by Touillaud et al [64]. This can be explained in several ways. First, an isoflavone from soy exerts weak oestrogen-like effect which has been suggested may in some cases stimulate BC metastasis. Under the physiological condition of elevated oestrogen levels, isoflavone competes poorly for the hormone receptor binding sites thereby and thus, exert additional proliferative effect [63]. For example, the additional tumour proliferative potential of genistein – a type of isoflavone at physiologically elevated oestrogen level has been demonstrated using mouse model [65]. To further validate this observation, an observed lessen tumour growth among soy-fed BALB/c mice upon injection with ER – human BC cells have been reported in Yan et al [50] and Kim et al [66].
Second, the physiological functions of oestrogen receptor in various organs (especially in the reproductive systems) are largely coordinated by proteins referred to as oestrogen receptor subtypes alpha (ERα) and beta (ERβ) [67]. The ERα and ERβ are primarily localized in (but not limited to) the mammary tissues and the granulose cells of the ovaries among women respectively [68]. Even though there exists a universal physiological function between these proteins, ERβ exerts an antagonistic potential on the ERα sensitivity – induced hyper-proliferation tumours (in signal transduction primarily activated by oestrogen) in mammary and uterus tissues [68, 69]. In absence of such oestrogen-sensitivities to activate ERα, the synergistic effect of ERβ and SI likely promotes a stronger combined anti-proliferative and anti-carcinogenic effect responsible for a potentially stronger protective association of SI-BC link among ER– BC subjects. The entire biological processes are not clearly understood and further studies are necessary.
Furthermore, previous reports [14, 18, 19] had mixed conclusions on the modifying effect of menopausal status on SI-BC risk. In our study, aside from observing the protective SI-BC link among women independent of menopausal status, the association appears stronger among premenopausal women. Baglia et al [7] in a recent longitudinal cohort reported similar findings predominantly among premenopausal women after 13.2year of follow-up. In that study, higher adult SI was protectively associated with postmenopausal BC risk. Also, Yamamoto et al [6] in a 9-year prospective study reported similar findings among postmenopausal women. Most women who participated in that study were already reaching (or at) postmenopausal status at baseline. Soy has been reported as a viable therapeutic regimen in slowing down menopausal symptoms [70, 71] and this perhaps offers a clue into the significance of menopausal status in the SI-BC risk association observed in our study. Independent of these differences, early long-term exposure to SI may be encouraged.
Collection of reports by ethnic origin in our study revealed SI had an inverse association with BC risk among Asian descent as other populations. Soy is a conspicuously consumed staple among Asians. Consumption of soy probably promotes intestinal capacities to significantly digest isoflavone in an effortlessly absorbable form. Metabolic activities of the intestinal microbiota are crucial to the physiological effect of isoflavones [72] and a pointer to the valuable role of soy on human health [73].
Despite the protective association of soy on BC risk has not enjoyed a consensus from previously published trials; it appears level-headed to promote soy consumption particularly early in the growth cycle given substantial evidence on the protective relationship of higher SI reported in observational studies. However, cross-cultural long-term trials are necessary (to appropriately discern the significance of HRS in the SI-BC link) taking into consideration functional components of soy, and the multifaceted interdependent signal transduction in BC angiogenesis.
4.1. Study limitations
Limitations evident in our study are worth mentioning. First, the estrogen receptor is not the only hormone receptor with an important role in breast cancer pathogenesis; our study only investigated the estrogen receptor. Other hormone receptors could not be explored due to the insufficiency of data. Second, most reports in this meta-analysis are case-control in design. Published cohort studies on SI-BC link taking into consideration previous history of SI stratified by hormone receptor status as well as those from non-Asian descents (most especially African population) are quite still limited. Our findings should be interpreted with caution particularly, in the light of limited longitudinal reports exploring the significance of hormone receptor status in the SI-BC relationship. Third, a significant amount of heterogeneity largely attributable to diversity in SI measurements exists in our study and substantial variation in adjustment for potential confounding variables across studies. The primary exposure in our study is soy. It is scarcely consumed singly but related to different soy-rich foods such as soybeans, tofu, soy milk, soy spread, etc. However, the sensitivity analysis and quality assessment of studies downplayed this limitation given that overall estimate of SI-BC link (though slightly attenuated) remained.
4.2. Conclusion and future directions
Higher SI is potentially associated with lessening of BC risk among women independent of oestrogen receptor BC phenotypes. The probable beneficial role of soy consumption in BC prevention and management stems from its functional component(s), and the inevitability of multi-centre longitudinal study with homogeneous quantitation of soy consumption (adjusting for intestinal proficiency for soy metabolization) is crucial in validating our findings.
Declarations
Author contribution statement
A. Okekunle: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper J. Gao: Performed the experiments; Contributed reagents, materials, analysis tools or data X. Wu: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data R. Feng: Analyzed and interpreted the data; Wrote the paper C. Sun: Conceived and designed the experiments; Wrote the paper.
Funding statement
This work was supported by the National Key R&D Program of China (2017YFC1307401), National Natural Science Foundation of China (81872616) and China Scholarship Council (2015BSZ778).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Akinkunmi Paul Okekunle, Email: akinokekunle@gmail.com.
Rennan Feng, Email: fengrennan@163.com.
Changhao Sun, Email: changhaosun2002@163.com.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Parkin D.M., Bray F.I., Devesa S.S. Cancer burden in the year 2000. The global picture. Eur. J. Cancer. 2001;37(Suppl 8):S4–66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A. Cancer statistics, 2005. CA Cancer J. Clin. 2005;55(1):10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 3.Chen W. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Fan L. Breast cancer in China. Lancet Oncol. 2014;15(7):e279–e289. doi: 10.1016/S1470-2045(13)70567-9. [DOI] [PubMed] [Google Scholar]
- 5.Horn-Ross P.L. Phytoestrogen intake and endometrial cancer risk. J. Natl. Cancer Inst. 2003;95(15):1158–1164. doi: 10.1093/jnci/djg015. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto S. Soy, isoflavones, and breast cancer risk in Japan. J. Natl. Cancer Inst. 2003;95(12):906–913. doi: 10.1093/jnci/95.12.906. [DOI] [PubMed] [Google Scholar]
- 7.Baglia M.L. The association of soy food consumption with the risk of subtype of breast cancers defined by hormone receptor and HER2 status. Int. J. Cancer. 2016;139(4):742–748. doi: 10.1002/ijc.30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caan B.J. Soy food consumption and breast cancer prognosis. Cancer Epidemiol. Biomarkers Prev. 2011;20(5):854–858. doi: 10.1158/1055-9965.EPI-10-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Touillaud M.S. No association between dietary phytoestrogens and risk of premenopausal breast cancer in a French cohort study. Canc. Epidemiol. Biomarkers Prev. 2006;15(12):2574–2576. doi: 10.1158/1055-9965.EPI-06-0543. [DOI] [PubMed] [Google Scholar]
- 10.Shu X.O. Soy food intake and breast cancer survival. J. Am. Med. Assoc. 2009;302(22):2437–2443. doi: 10.1001/jama.2009.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang X. Effect of soy isoflavones on breast cancer recurrence and death for patients receiving adjuvant endocrine therapy. CMAJ. 2010;182(17):1857–1862. doi: 10.1503/cmaj.091298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang F.F. Dietary isoflavone intake and all-cause mortality in breast cancer survivors: the Breast Cancer Family Registry. Cancer. 2017;123(11):2070–2079. doi: 10.1002/cncr.30615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y.F. Positive effects of soy isoflavone food on survival of breast cancer patients in China. Asian Pac. J. Cancer Prev. 2012;13(2):479–482. doi: 10.7314/apjcp.2012.13.2.479. [DOI] [PubMed] [Google Scholar]
- 14.Trock B.J., Hilakivi-Clarke L., Clarke R. Meta-analysis of soy intake and breast cancer risk. J. Natl. Cancer Inst. 2006;98(7):459–471. doi: 10.1093/jnci/djj102. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y.C. Meta-analysis of studies on breast cancer risk and diet in Chinese women. Int. J. Clin. Exp. Med. 2015;8(1):73–85. [PMC free article] [PubMed] [Google Scholar]
- 16.Nechuta S.J. Soy food intake after diagnosis of breast cancer and survival: an in-depth analysis of combined evidence from cohort studies of US and Chinese women. Am. J. Clin. Nutr. 2012;96(1):123–132. doi: 10.3945/ajcn.112.035972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong X., Zhang C. Soy food intake and breast cancer risk: a meta-analysis. Wei Sheng Yan Jiu. 2012;41(4):670–676. [PubMed] [Google Scholar]
- 18.Dong J.Y., Qin L.Q. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: a meta-analysis of prospective studies. Breast Cancer Res. Treat. 2011;125(2):315–323. doi: 10.1007/s10549-010-1270-8. [DOI] [PubMed] [Google Scholar]
- 19.Fritz H. Soy, red clover, and isoflavones and breast cancer: a systematic review. PloS One. 2013;8(11) doi: 10.1371/journal.pone.0081968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin L.Q. Soyfood intake in the prevention of breast cancer risk in women: a meta-analysis of observational epidemiological studies. J. Nutr. Sci. Vitaminol. (Tokyo) 2006;52(6):428–436. doi: 10.3177/jnsv.52.428. [DOI] [PubMed] [Google Scholar]
- 21.Stroup D.F. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. J. Am. Med. Assoc. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 22.Wells G.A. 2014. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [cited 2016 May]; Available from: [Google Scholar]
- 23.Hedelin M. Dietary phytoestrogens are not associated with risk of overall breast cancer but diets rich in coumestrol are inversely associated with risk of estrogen receptor and progesterone receptor negative breast tumors in Swedish women. J. Nutr. 2008;138(5):938–945. doi: 10.1093/jn/138.5.938. [DOI] [PubMed] [Google Scholar]
- 24.Key T.J. Soya foods and breast cancer risk: a prospective study in Hiroshima and Nagasaki, Japan. Br. J. Cancer. 1999;81(7):1248–1256. doi: 10.1038/sj.bjc.6690837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S.A. Adolescent and adult soy food intake and breast cancer risk: results from the Shanghai Women's Health Study. Am. J. Clin. Nutr. 2009;89(6):1920–1926. doi: 10.3945/ajcn.2008.27361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Travis R.C. A prospective study of vegetarianism and isoflavone intake in relation to breast cancer risk in British women. Int. J. Cancer. 2008;122(3):705–710. doi: 10.1002/ijc.23141. [DOI] [PubMed] [Google Scholar]
- 27.Wu A.H. Soy intake and breast cancer risk in Singapore Chinese Health Study. Br. J. Cancer. 2008;99(1):196–200. doi: 10.1038/sj.bjc.6604448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan J.M. Diet and breast cancer in Shanghai and Tianjin, China. Br. J. Cancer. 1995;71(6):1353–1358. doi: 10.1038/bjc.1995.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wada K. Soy isoflavone intake and breast cancer risk in Japan: from the Takayama study. Int. J. Cancer. 2013;133(4):952–960. doi: 10.1002/ijc.28088. [DOI] [PubMed] [Google Scholar]
- 30.Woo H.D. Differential influence of dietary soy intake on the risk of breast cancer recurrence related to HER2 status. Nutr. Cancer. 2012;64(2):198–205. doi: 10.1080/01635581.2012.635261. [DOI] [PubMed] [Google Scholar]
- 31.Cho Y.A. Effect of dietary soy intake on breast cancer risk according to menopause and hormone receptor status. Eur. J. Clin. Nutr. 2010;64(9):924–932. doi: 10.1038/ejcn.2010.95. [DOI] [PubMed] [Google Scholar]
- 32.Dai Q. Population-based case-control study of soyfood intake and breast cancer risk in Shanghai. Br. J. Cancer. 2001;85(3):372–378. doi: 10.1054/bjoc.2001.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horn-Ross P.L. Phytoestrogen consumption and breast cancer risk in a multiethnic population: the Bay Area Breast Cancer Study. Am. J. Epidemiol. 2001;154(5):434–441. doi: 10.1093/aje/154.5.434. [DOI] [PubMed] [Google Scholar]
- 34.Kim M.K. Dietary intake of soy protein and tofu in association with breast cancer risk based on a case-control study. Nutr. Cancer. 2008;60(5):568–576. doi: 10.1080/01635580801966203. [DOI] [PubMed] [Google Scholar]
- 35.Lee M.M. Breast cancer and dietary factors in Taiwanese women. Cancer Causes Control. 2005;16(8):929–937. doi: 10.1007/s10552-005-4932-9. [DOI] [PubMed] [Google Scholar]
- 36.Li W. Dietary and other risk factors in women having fibrocystic breast conditions with and without concurrent breast cancer: a nested case-control study in Shanghai, China. Int. J. Cancer. 2005;115(6):981–993. doi: 10.1002/ijc.20964. [DOI] [PubMed] [Google Scholar]
- 37.Shannon J. Food and botanical groupings and risk of breast cancer: a case-control study in Shanghai, China. Cancer Epidemiol. Biomarkers Prev. 2005;14(1):81–90. [PubMed] [Google Scholar]
- 38.Suzuki T. Effect of soybean on breast cancer according to receptor status: a case-control study in Japan. Int. J. Cancer. 2008;123(7):1674–1680. doi: 10.1002/ijc.23644. [DOI] [PubMed] [Google Scholar]
- 39.Wu A.H. Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis. 2002;23(9):1491–1496. doi: 10.1093/carcin/23.9.1491. [DOI] [PubMed] [Google Scholar]
- 40.Wu A.H. Dietary patterns and breast cancer risk in Asian American women. Am. J. Clin. Nutr. 2009;89(4):1145–1154. doi: 10.3945/ajcn.2008.26915. [DOI] [PubMed] [Google Scholar]
- 41.Wu A.H. Tofu and risk of breast cancer in Asian-Americans. Canc. Epidemiol. Biomarkers Prev. 1996;5(11):901–906. [PubMed] [Google Scholar]
- 42.Zhu Yan-Yun, Zhou L., Shun-chang J., Liang-zhi X. Relationship between soy food intake and breast cancer in China. Asian Pac. J. Cancer Prev. 2011;12:4. [PubMed] [Google Scholar]
- 43.Zaineddin A.K. The association between dietary lignans, phytoestrogen-rich foods, and fiber intake and postmenopausal breast cancer risk: a German case-control study. Nutr. Cancer. 2012;64(5):652–665. doi: 10.1080/01635581.2012.683227. [DOI] [PubMed] [Google Scholar]
- 44.Ziaei S., Halaby R. Dietary isoflavones and breast cancer risk. Medicines (Basel) 2017;4(2) doi: 10.3390/medicines4020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim J. Fermented and non-fermented soy food consumption and gastric cancer in Japanese and Korean populations: a meta-analysis of observational studies. Cancer Sci. 2011;102(1):231–244. doi: 10.1111/j.1349-7006.2010.01770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu J. Dietary protein sources and incidence of breast cancer: a dose-response meta-analysis of prospective studies. Nutrients. 2016;8(11) doi: 10.3390/nu8110730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao T.-T. Dietary isoflavones or isoflavone-rich food intake and breast cancer risk: a meta-analysis of prospective cohort studies. Clin. Nutr. 2019;38(1):136–145. doi: 10.1016/j.clnu.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 48.Food labeling: health claims; soy protein and coronary heart disease. Food Drug Administration HHS Final Rule. Fed. Regist. 1999;64(206):57700–57733. [PubMed] [Google Scholar]
- 49.Lima A. Proteins in soy might have a higher role in cancer prevention than previously expected: soybean protein fractions are more effective MMP-9 inhibitors than non-protein fractions, even in cooked seeds. Nutrients. 2017;9(3) doi: 10.3390/nu9030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan L., Li D., Yee J.A. Dietary supplementation with isolated soy protein reduces metastasis of mammary carcinoma cells in mice. Clin. Exp. Metastasis. 2002;19(6):535–540. doi: 10.1023/a:1020377311532. [DOI] [PubMed] [Google Scholar]
- 51.Farvid M.S. Dietary fiber intake in young adults and breast cancer risk. Pediatrics. 2016;137(3) doi: 10.1542/peds.2015-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen S. Dietary fibre intake and risk of breast cancer: a systematic review and meta-analysis of epidemiological studies. Oncotarget. 2016;7(49):80980–80989. doi: 10.18632/oncotarget.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kerwin S.M. Soy saponins and the anticancer effects of soybeans and soy-based foods. Curr. Med. Chem. Anticancer Agents. 2004;4(3):263–272. doi: 10.2174/1568011043352993. [DOI] [PubMed] [Google Scholar]
- 54.Valachovicova T. Soy isoflavones suppress invasiveness of breast cancer cells by the inhibition of NF-kappaB/AP-1-dependent and -independent pathways. Int. J. Oncol. 2004;25(5):1389–1395. [PubMed] [Google Scholar]
- 55.Le Bail J.C. Effects of phytoestrogens on aromatase, 3beta and 17beta-hydroxysteroid dehydrogenase activities and human breast cancer cells. Life Sci. 2000;66(14):1281–1291. doi: 10.1016/s0024-3205(00)00435-5. [DOI] [PubMed] [Google Scholar]
- 56.Zafar A., Singh S., Naseem I. Cytotoxic activity of soy phytoestrogen coumestrol against human breast cancer MCF-7 cells: insights into the molecular mechanism. Food Chem. Toxicol. 2017;99:149–161. doi: 10.1016/j.fct.2016.11.034. [DOI] [PubMed] [Google Scholar]
- 57.Barnes M. Tumor induction following intraoperative radiotherapy: late results of the National Cancer Institute canine trials. Int. J. Radiat. Oncol. Biol. Phys. 1990;19(3):651–660. doi: 10.1016/0360-3016(90)90492-3. [DOI] [PubMed] [Google Scholar]
- 58.Xiao Y. Comprehensive evaluation of the role of soy and isoflavone supplementation in humans and animals over the past two decades. Phytother Res. 2017 doi: 10.1002/ptr.5966. [DOI] [PubMed] [Google Scholar]
- 59.Adlercreutz H., Mazur W. Phyto-oestrogens and Western diseases. Ann. Med. 1997;29(2):95–120. doi: 10.3109/07853899709113696. [DOI] [PubMed] [Google Scholar]
- 60.Taylor C.K. The effect of genistein aglycone on cancer and cancer risk: a review of in vitro, preclinical, and clinical studies. Nutr. Rev. 2009;67(7):398–415. doi: 10.1111/j.1753-4887.2009.00213.x. [DOI] [PubMed] [Google Scholar]
- 61.DiSilvestro R.A. Soy isoflavone supplementation elevates erythrocyte superoxide dismutase, but not plasma ceruloplasmin in postmenopausal breast cancer survivors. Breast Cancer Res. Treat. 2005;89(3):251–255. doi: 10.1007/s10549-004-2227-6. [DOI] [PubMed] [Google Scholar]
- 62.Khan S.A. Soy isoflavone supplementation for breast cancer risk reduction: a randomized phase II trial. Cancer Prev. Res. (Phila) 2012;5(2):309–319. doi: 10.1158/1940-6207.CAPR-11-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stubert J., Gerber B. Isoflavones - mechanism of action and impact on breast cancer risk. Breast Care (Basel) 2009;4(1):22–29. doi: 10.1159/000200980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Touillaud M.S. Effect of dietary intake of phytoestrogens on estrogen receptor status in premenopausal women with breast cancer. Nutr. Cancer. 2005;51(2):162–169. doi: 10.1207/s15327914nc5102_6. [DOI] [PubMed] [Google Scholar]
- 65.Hwang C.S. Isoflavone metabolites and their in vitro dual functions: they can act as an estrogenic agonist or antagonist depending on the estrogen concentration. J. Steroid Biochem. Mol. Biol. 2006;101(4-5):246–253. doi: 10.1016/j.jsbmb.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 66.Kim H.A., Jeong K.S., Kim Y.K. Soy extract is more potent than genistein on tumor growth inhibition. Anticancer Res. 2008;28(5A):2837–2841. [PubMed] [Google Scholar]
- 67.Paterni I. Estrogen receptors alpha (ERalpha) and beta (ERbeta): subtype-selective ligands and clinical potential. Steroids. 2014;90:13–29. doi: 10.1016/j.steroids.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leitman D.C. Regulation of specific target genes and biological responses by estrogen receptor subtype agonists. Curr. Opin. Pharmacol. 2010;10(6):629–636. doi: 10.1016/j.coph.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Setchell K.D., Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J. Nutr. 1999;129(3):758S–767S. doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- 70.Han K.K. Benefits of soy isoflavone therapeutic regimen on menopausal symptoms. Obstet. Gynecol. 2002;99(3):389–394. doi: 10.1016/s0029-7844(01)01744-6. [DOI] [PubMed] [Google Scholar]
- 71.Chen M.N., Lin C.C., Liu C.F. Efficacy of phytoestrogens for menopausal symptoms: a meta-analysis and systematic review. Climacteric J. Int. Menopause Soc. 2015;18(2):260–269. doi: 10.3109/13697137.2014.966241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Landete J.M. Probiotic bacteria for healthier aging: immunomodulation and metabolism of phytoestrogens. BioMed Res. Int. 2017;2017 doi: 10.1155/2017/5939818. 5939818-5939818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kolátorová L., Lapčík O., Stárka L. Phytoestrogens and the intestinal microbiome. Physiol. Res. 2018;67(Suppl 3):S401–S408. doi: 10.33549/physiolres.934022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.