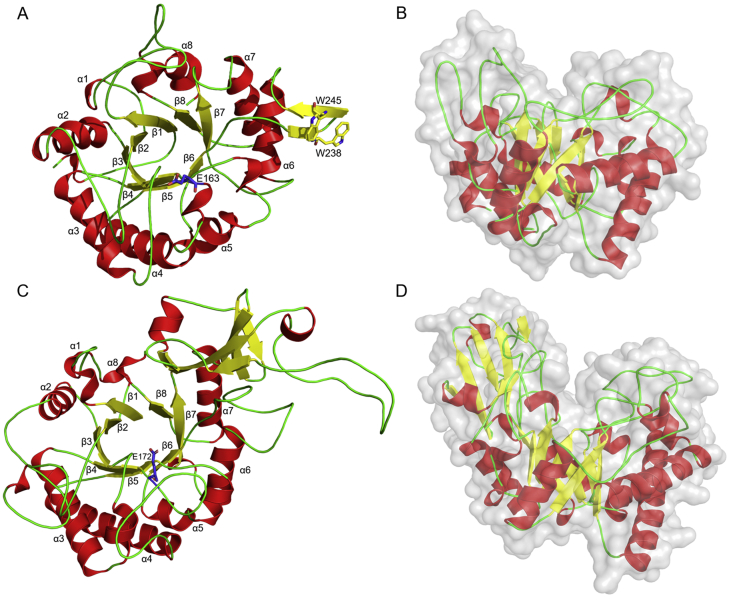
Figure 7.
The overall 3D structural models (top view) of LmChiA and LmChiB catalytic domains with an (α/β)8 TIM barrel fold shown in ribbon drawings (A and C). The catalytic glutamates (E163 in LmChiA, and E172 in LmChiB) are positioned between β-strand 4 and α-helix 4 as shown by blue sticks. Two exposed tryptophan residues (W238 and W245) involved in substrate binding are shown by yellow sticks. Molecular surfaces with ribbon representation of LmChiA and LmChiB catalytic domains (side view) are shown in B and D. An α-helix is represented by red, β-strand by yellow, and loop by green.