Abstract
Background
Right heart catheterization (RHC) is the gold-standard in the diagnosis of pulmonary hypertension (PH) but at the cost of procedure-related complications. We sought to determine the comparative accuracy of RHC versus non-invasive imaging techniques such as computed tomography (CT), magnetic resonance imaging (MRI), and transthoracic echocardiography (TTE).
Methods
Pulmonary hypertension was defined as a mean pulmonary artery pressure (mPAP) of>20 mmHg. Multiple databases were queried for relevant articles. Raw data were pooled using a bivariate model to calculate the measures of diagnostic accuracy and to estimate Hierarchical Summary Receiver Operating Characteristic (HSROC) on Stata 13.
Results
A total of 51 studies with a total patient population of 3947 were selected. The pooled sensitivity and specificity of MRI for diagnosing PH was 0.92(95% confidence interval (CI) 0.88–0.96) and 0.86 (95% CI, 0.77–0.95), respectively. The net sensitivities for CT scan and TTE were 0.79 (95% CI 0.72–0.89) and 0.85 (95% CI 0.83–0.91), respectively. The overall specificity was 0.82 (0.76–0.92) for the CT scan and 0.71 (95% CI 0.61–0.84) for TTE. The diagnostic odds ratio (DOR) for MRI was 124 (95% CI 36–433) compared to 30 (95% CI 11–78) and 24 (95% 11–38) for CT scan and TTE, respectively. Chi-squared (x2) test showed moderate heterogeneity on the test for equality of sensitivities and specificities.
Conclusions
MRI has the highest sensitivity and specificity compared to CT and TTE. MRI can potentially serve as a surrogate technique to RHC for the diagnosis of PH.
1. Introduction
RHC is a gold standard for the diagnosis of pH by measuring mean pulmonary arterial systolic pressure (mPASP) [1]. Although RHC has the highest diagnostic accuracy, it comes at the cost of invasive procedure, catheter-related complications, and occasionally risk of data misinterpretation due to errors in catheter placement [2]. Traditionally, less invasive techniques, including TTE, have been an area of interest for diagnosis of PH [3].
TTE is extensively used for the screening and diagnosis of PH. It might allow for accurate estimates of mPASP, but with moderate precision, which makes it an appropriate approach for population studies rather than for individual patients [4]. Recently, CT and MRI has been gaining traction as a non-invasive alternative to RHC. The study by Shen et al. reported that CT scan have a sensitivity and specificity of 0.79–0.83 and 0.74–0.81, respectively [5], [6]. Despite the recent advances, the comparative accuracy of less invasive methods (TTE, CT, and MRI) against RHC remains an area of uncertainty.
2. Methods:
2.1. Search strategy
A literature search for relevant articles was performed from inception to April 15, 2020, using PubMed, Ovid, Embase, clinicaltrials.gov, and Cochrane databases. There was no language or time restriction placed. The search strategies included various combinations of keywords and medical subject headings (MeSH) to generate four subsets of citations: magnetic resonance imaging (MRI), computed tomography (CT), transthoracic echocardiography (TTE) and pulmonary hypertension (PH). The terms from all subsets were combined in 1:1 combination using Boolean operators, and results from all possible combinations were downloaded into an EndNote library. Based on our research question, articles from the reference lists relevant to the clinical question were also screened by an independent author.
2.2. Selection criteria and study collection
All observational (retrospective/prospective) studies and randomized control trials (RCT) were evaluated. Articles comparing the diagnostic accuracy of MRI, CT, or TEE with RHC were included in the study. Inclusion criteria for study articles were as follows: (1) original articles published in English; (2) it included subjects who had mPAP, mPAD or PA:A ratio measurement for detection of PH; (3) surrogate markers for PH such as RV strain or tricuspid regurgitation was assessed; (4) patients were in stable condition; and (5) articles reported sufficient raw data to calculate sensitivity and specificity. (6) The most common criteria of TTE used to define PH included pulmonary artery systolic and mean pressure, tricuspid regurgitation, right ventricular systolic pressure, right ventricular diameter, and right ventricular free wall strain. CT and MRI also used interventricular septum curvature, ratio of interventricular septum curvature and left ventricular free wall, pulmonary artery pulsatility, pulmonary artery distensibility, ventricular mass index, pulmonary artery stiffness, and dilation of the main pulmonary artery as diagnostic criterias. Papers with insufficient data, review articles, case reports, editorials, and conference papers were excluded. Pulmonary hypertension was defined as a mean pulmonary artery pressure (mPAP) of>20 mmHg.
2.3. Data extractions
Five authors independently screened titles and abstracts of all the articles for relevance, and the sixth author selected articles which met the inclusion criteria. Full text articles that were potentially relevant to the study was screened by the seventh author to confirm eligibility. Baseline characteristics of the included population were reviewed, and data were collected for quality assessment to ensure study cohorts were statistically comparable. Data was finally extracted into the combined Excel sheet. Disagreements were resolved by mutual consensus and after a detailed group discussion.
2.4. Data and quality analysis
Raw data regarding the true and false positives and true and false negatives of each included study was obtained, and the combined measures of test accuracy [i.e., sensitivity, specificity, and diagnostic odds ratio (DOR)] for all non-invasive imaging modalities were calculated, keeping RHC a standard. A bivariate model was obtained from data fitting. The parameters from the bivariate model were transformed into Hierarchical Summary Receiver Operating Characteristic (HSROC) estimates. The area under the summary HSROC curve (AUC) was calculated to reveal the probability of accurately ranked diagnostic test values for a random pair of subjects (one with disease and one without disease). Chi-squared (x2) tests were conducted to evaluate the heterogeneity of sensitivities and specificities among all studies. Subgroup analysis based on the diagnostic threshold cutoff points of the index test was done to explore the potential heterogeneity factors using meta-regression analysis. Quality of the included studies was assessed for the potential bias and applicability concerns using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) standard questionnaire. The statistical analysis was performed using the diagnostic accuracy statistical model on the Stata and R mad package.
2.5. Quality of included studies
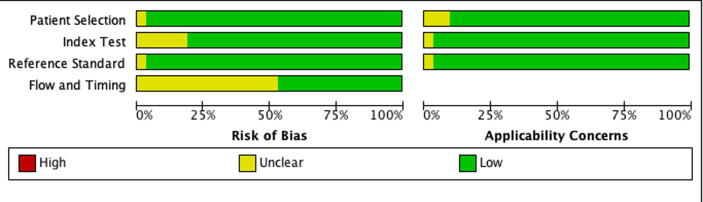
Based on the QUADAS-2 tool, the overall risk of bias and applicability concerns based on patient selection, index and reference standard was low. (Fig. 1) In the study by Cotton et al., the patient selection bias was of unclear concern. The risk of bias associated with flow and timing was of unclear concern due to inadequate reporting across most studies. (Fig. 1) The optimal timing between the index test (TTE, CT or MRI) and the reference standard (RHC) was uncertain across most articles. The impact of inadequate reporting on the interpretation of their respective results is uncertain.
Fig. 1.
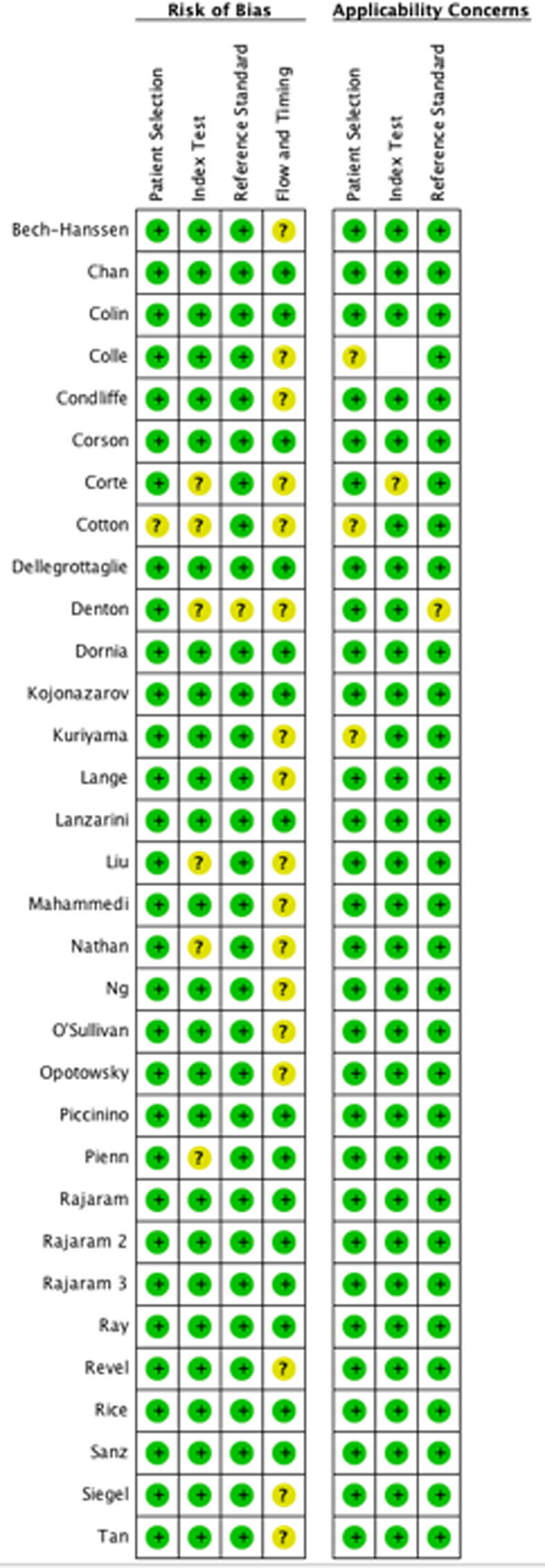
Summary and Detailed Quality assessment of the included studies.
3. Results
3.1. Search results and study selection
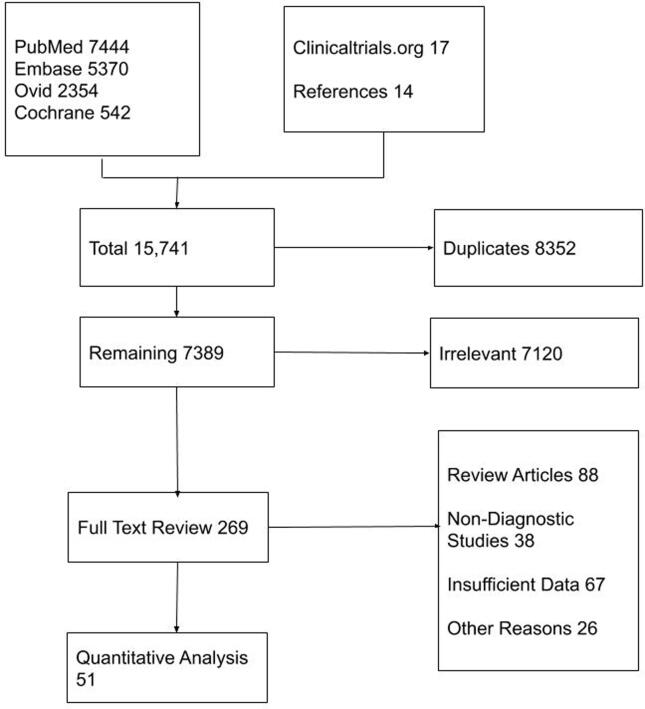
The initial search on included databases revealed 15,741 articles. After the removal of irrelevant and duplicate items, only 269 articles were deemed relevant for full-text review. We further excluded 219 articles based on our selection criteria; only 51 studies were included for final analysis. The Prisma flow diagram is shown in Fig. 2.
Fig. 2.
PRISMA flow diagram of the included studies.
A total of 51 studies comprising 3947 patients, were included in the study. The mean age of the included patients was 56 years, with an average male population of 46%. The detailed characteristics of the included studies are summarized in supplementary table 1.
3.2. Pooled sensitivity and specificity Results:
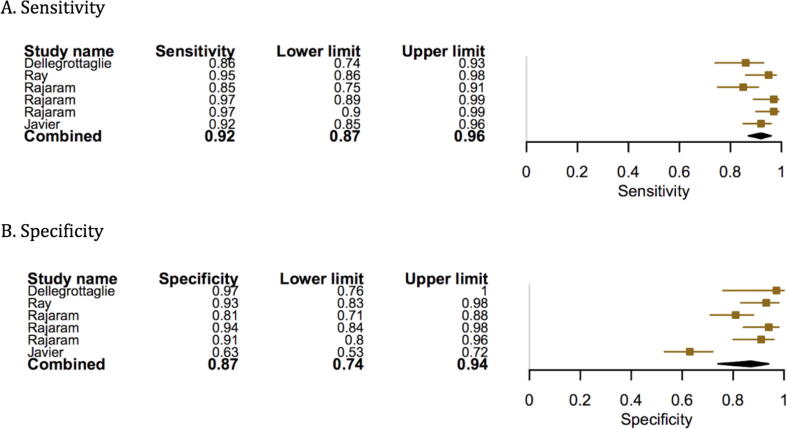
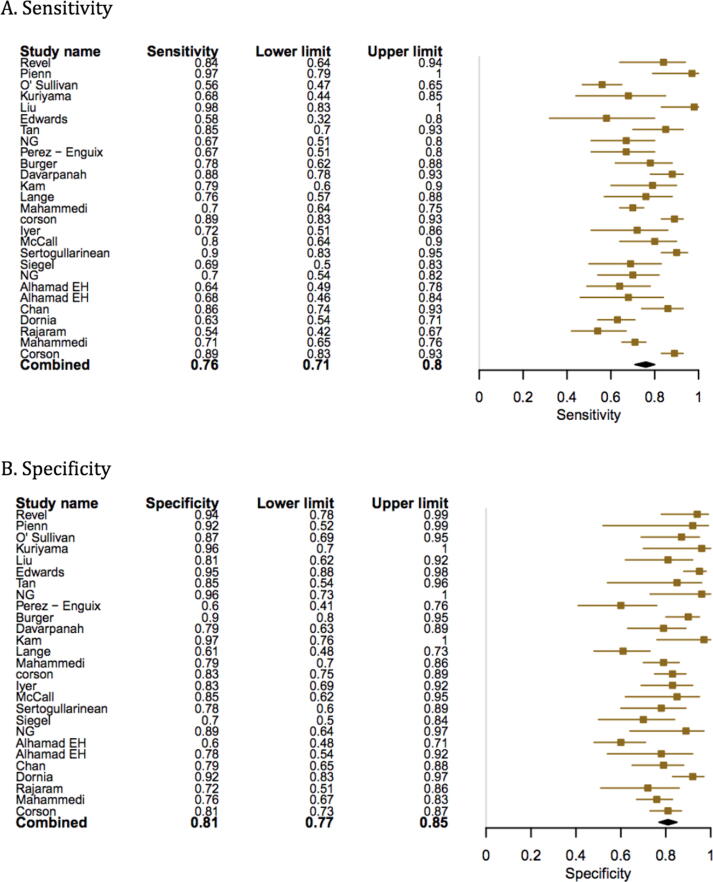
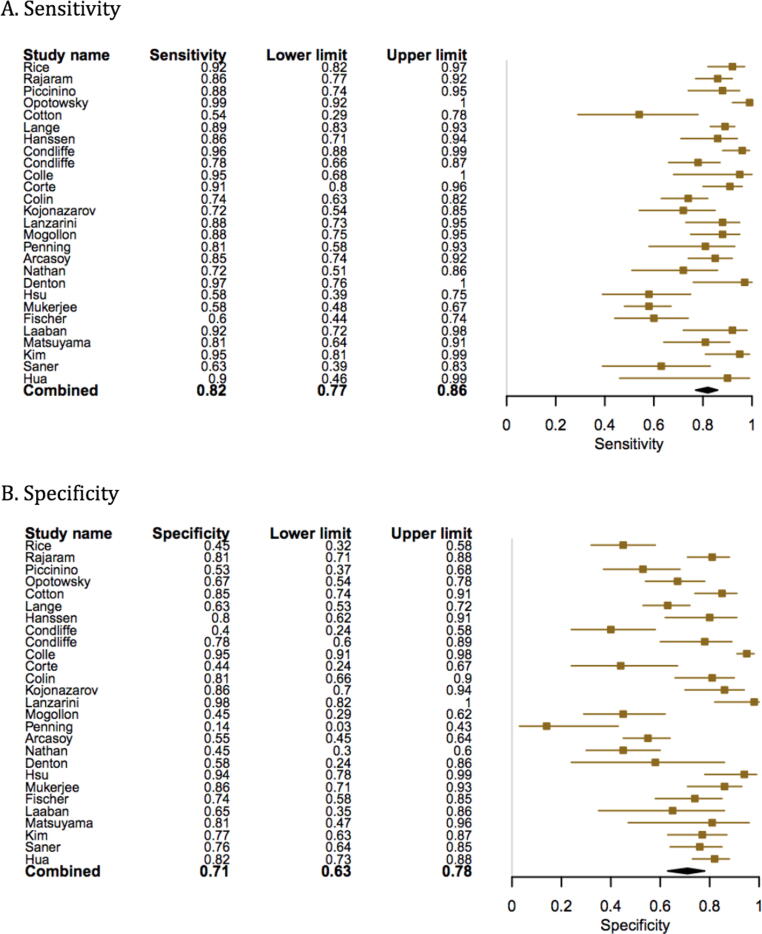
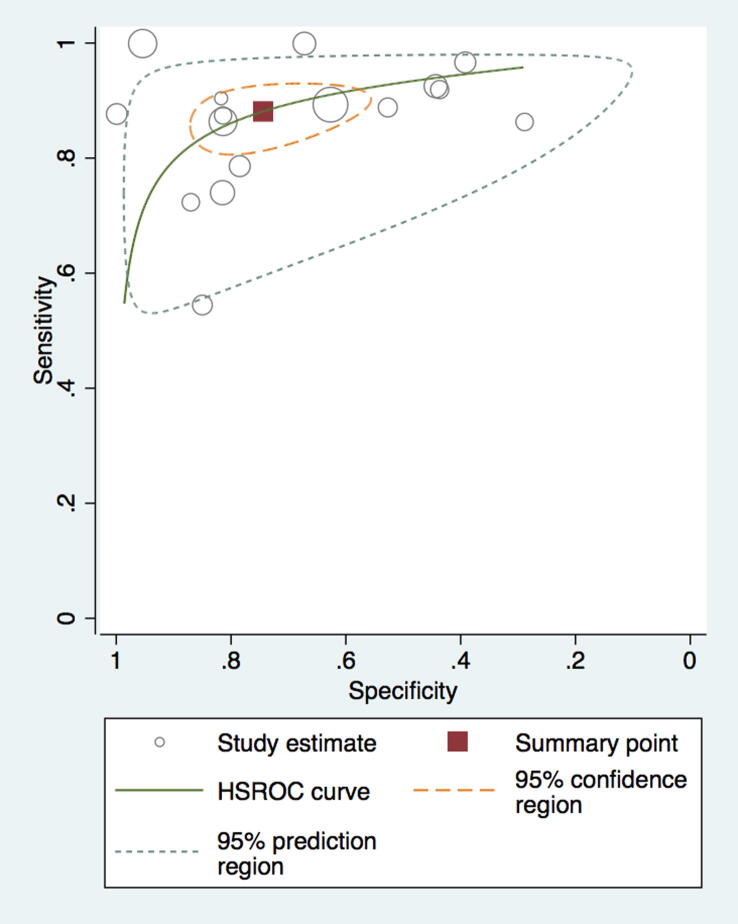
Compared to RHC, the pooled sensitivity and specificity of MRI for diagnosing PH was 0.92 (95% confidence interval (CI) 0.88–0.96) and 0.86 (95% CI, 0.77–0.95). (Fig. 3, Fig. 4) The sensitivity and specificity of CT scan was 0.79 (95% CI, 0.72–0.89) and 0.82 (95% CI, 0.76–0.92) (Fig. 5, Fig. 6). TTE showed a sensitivity and specificity of 0.85 (95% CI, 0.83–0.91) and 0.71 (95% CI, 0.61–0.84) (Fig. 7, Fig. 8).
Fig. 3.
Forest plot showing the individual and pooled sensitivity and specificity of MRI.
Fig. 4.
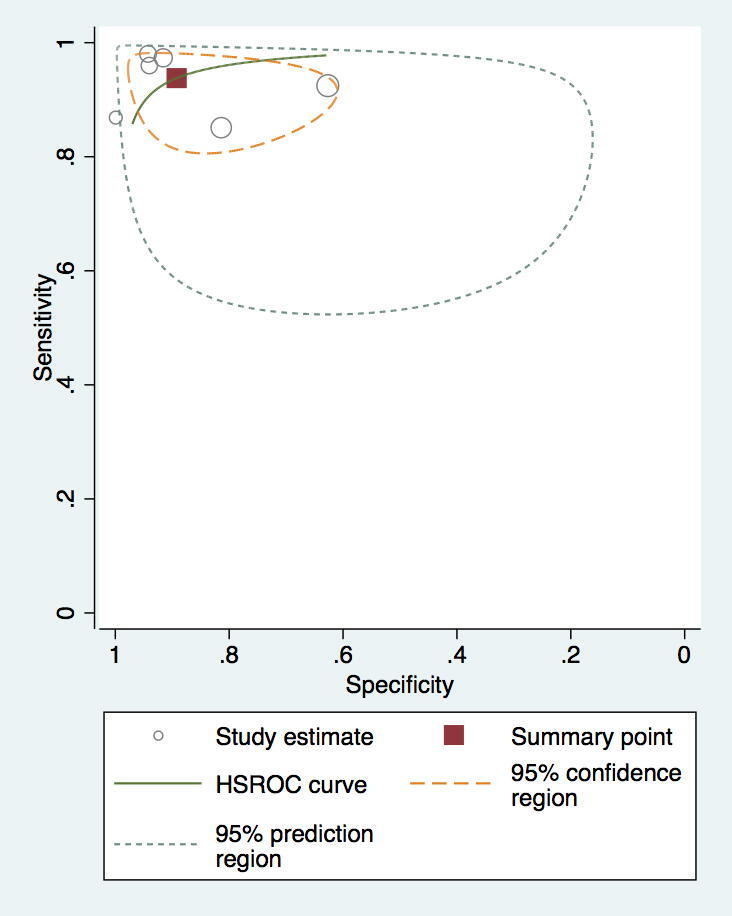
ROC demonstrating a higher sensitivity and specificity for MRI compared to RHC.
Fig. 5.
Forest plot showing the individual and pooled sensitivity and specificity of CT.
Fig. 6.
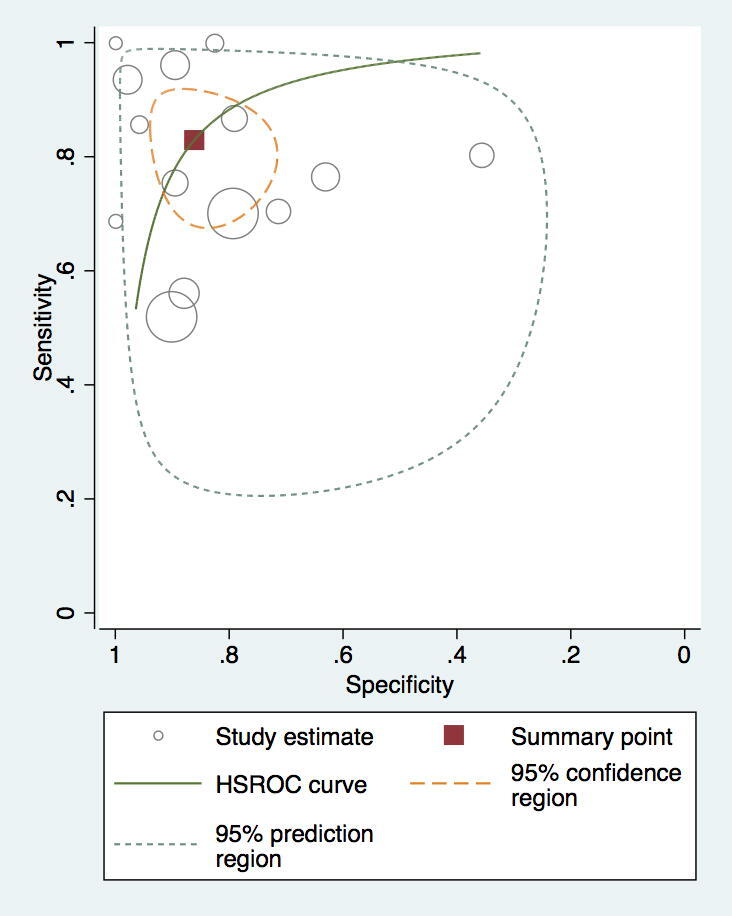
ROC demonstrating a higher sensitivity and specificity for CT compared to RHC.
Fig. 7.
Forest plot showing the individual and pooled sensitivity and specificity of TTE.
Fig. 8.
ROC demonstrating a higher sensitivity and specificity for TTE compared to RHC.
3.3. Subgroup Analysis:
A subgroup analysis based on the matched inclusion criteria and diagnostic parameters of PH was also performed. The subgroup analysis mirrored the overall findings of the pooled analysis with a few exceptions. In contrast to the pooled results of TTE, the sensitivity and specificity values were 0.92 and 0.45, when RV free wall strain was used as a diagnostic criteria. On the other hand, the sensitivity and specificity of different parameters used for defining PH based on CT scan ranged from (0.65–0.84) and (0.78–0.90), respectively, which were comparable to the corresponding pooled results. The detailed subgroup diagnostic accuracy values are shown in Table 1.
Table 1.
Subgroup diagnostic accuracy of CT and TTE based on the inclusion criterias.
| Criteria | Imaging | No. of studies | Mean sensitivity | Mean specificity |
|---|---|---|---|---|
| Pulmonary artery systolic pressure (PASP) | TTE | 12 | 0.78 | 0.68 |
| Right ventricular systolic pressure (RVSP) | TTE | 6 | 0.88 | 0.62 |
| Tricuspid regurgitant jet (TRJ) | TTE | 6 | 0.81 | 0.84 |
| RV free wall strain | TTE | 1 | 0.92 | 0.45 |
| Right ventricle diameter (RVd) | TTE | 2 | 0.79 | 0.77 |
| Pulmonary artery diameter (PAd) | CT | 15 | 0.71 | 0.78 |
| RV diameter | CT | 2 | 0.84 | 0.90 |
| RV wall thickness | CT | 3 | 0.74 | 0.81 |
| PA diameter/AA diameter | CT | 5 | 0.65 | 0.79 |
3.4. Chi-squared testing results
The heterogeneity among the outcomes of the included studies was moderate through all comparisons. The Chi-squared (x2) test for the sensitivity and specificity of MRI was 13 and 38, with a p=<0.1. The x2-test for the sensitivity and specificity of CT was 131 and 73 with a p=< 0.1 while it was 47 and 162 (p=< 0.1), respectively.
3.5. Likelihood and diagnostic odds ratio results
The positive likelihood ratios (PLR) for MRI, CT and TTE were 8.7 ± 3.5 (95% CI 3.93–19.27), 6.0 ± 0.8 (95% CI 3.3–10.6) and 3.4 ± 0.8 (95% CI 2.23–5.31) respectively. The negative likelihood ratios (NLR) for MRI, CT and TTE was 0.069 ± 0.2 (95% CI 0.035–0.13), 0.19 ± 0.01 (0.11–0.33) and 0.15 ± 0.03 (95% CI 0.11–0.22) respectively. The diagnostic odds ratio (DOR) for MRI was highest at 124 (95% CI, 36–433) compared to 30 (95% CI, 11–78) and 24 (95% CI, 11–38) for CT and TTE, respectively
4. Discussion
To the best of our knowledge, this is the first meta-analysis comparing multiple noninvasive modalities with RHC for the diagnosis of pulmonary hypertension. This is also the most updated meta-analysis with the largest number of studies and the largest sample size in each noninvasive diagnostic test category. Our results revealed that all three noninvasive imaging modalities might have a predictive value for the diagnosis of pulmonary hypertension (PH). On pooled analysis, MRI demonstrated the greatest diagnostic accuracy as signified by a large area under the curve on Receiver Operating Characteristic (ROC). The sensitivity and specificity of TTE and CT scan were also comparable to right heart catheterization (RHC), however, was relatively lower compared to MRI. Chi-squared testing for the evaluation of heterogeneity resulted in moderate heterogeneity, inferring that the results demonstrated likely reflect the true ability of other imaging modalities in the prediction of pH as compared to RHC. Though all three imaging modalities demonstrated a positive and significant relation to RHC, the likelihood ratios (LR) and diagnostic odds ratio (DOR) substantially favored MRI than CT and TTE in comparison to RHC.
PH results in dilatation of the right-sided heart chambers and subsequent tricuspid regurgitation [7]. These two phenomena play a critical role in the estimation of PH via noninvasive tests [8]. A mean pulmonary arterial pressure (PAP > 20 mmHg) is considered a diagnostic of PH when measured at rest in the supine position. However, a variety of parameters and cutoff were found to be suggestive of PH, explaining the heterogeneity among the included studies [9]. The suggestive findings for PH on MRI included ventricular mass index (VMI) and right atrial (RA) pulsatility. VMI is calculated by dividing the right ventricular mass by that of the left ventricle mass (VMI = RVM/LVM) [10]. The reported sensitivity and specificity of VMI is 84% and 82%, respectively [11]. A study by Seba et al. found VMI assessment of PH by MRI has a higher accuracy as compared to pressure assessment of TTE [12]. A meta-analysis by Wang et al. also used VMI as a measure and found to have high pooled sensitivity (84%) and specificity (82%)[11]. Our meta-analysis included 6 studies to assess the diagnostic accuracy of MRI using both RA pulsatility (below 40%) and VMI (>0.45). Other suggestive measures included pulmonary artery (PA) to ascending aorta (AO) ratio (PA/AO). This ratio is an indirect measure of interventricular septal flattening during systole and correlates with PASP [13]. Our meta-analysis reports higher results than wang et al. and yielded a pooled sensitivity and specificity of MRI as 0.92 and 0.86, respectively. Regardless of the measurement used, MRI is found to be valuable in terms of prognosis and follow up after treatment [14], [15], [16].
By contrast, TTE is the most convenient and initial noninvasive screening tool. The including measurements of TTE for PH diagnosis and assessment include pulmonary artery regurgitant jet method, measurement of the tricuspid annular plane systolic excursion (TAPSE), two-dimensional strain tissue Doppler echocardiography, the speckle tracking method, acceleration time across the pulmonic valve and the tricuspid regurgitant jet velocity (TRV) [17], [18], [19], [20], [21]. TRV is used to calculate the ePASP using RAP, which can be clinically estimated from jugular venous pressure (JVP) using a fixed value of 10 mm Hg, and the diameter and collapse of the inferior vena cava (IVC) during spontaneous respiration [18], [22], [23], [24]. ESC and ERS recommend using peak TRV alongside other echocardiographic findings suggestive of PH such as right ventricle/left ventricle basal diameter ratio > 1.0, right ventricular outflow Doppler acceleration time < 105 ms and/or mid-systolic notching & right atrial end-systole area > 18 cm2 [25]. Our review showed that the study by Condliffe et al. used TRV to calculate the tricuspid gradient (TG), while Collin et al. and Rajaram et. el. used TR gradient (cut off of > 27 mmHg and>40) for diagnosing PH [26], [27], [28]. Surrender et al., used sPAP as a measure of assessment of PH with a cutoff range ≥ 30 mmHg [4]. The mPASP was calculated based on the maximum velocity of the tricuspid regurgitant jet method (TRJ + RAP) (estimated using JVP/IVC collapse). Zhang et al. included 6 studies, the measure used for the diagnosis of PH was RVSP and PASP, demonstrating intermediate sensitivity results compared to RHC [29]. A prior systematic review published in 2010, assessed 29 studies, comparing TTE for screening and diagnosis of PH. PASP measurement had a correlation of about 0.7 with RHC, with pooled sensitivity and specificity of 0.83 and 0.72, respectively [4]. Another meta-analysis examining two variables on CT, PA diameter and PA:A ratio was published by Shen et al. in 2014. The reported sensitivities and specificities for the former was found to be 0.79 and 0.83, while for the later, it was 0.74 and 0.81, respectively [5]. Of note, PA diameter was also found to correlate with prognosis in patients with chronic respiratory illness, e.g., bronchiectasis [6].
On the contrary, our analysis included the largest population from a total of 27 studies comparing TTE and RHC. Most included studies used TRJ and RAP to calculate mPASP. Of the included studies, Opotowsky et al. used multiple parameters including RV dilatation, prediction rule (ranges from −2 to + 2) and mid systolic notch or acceleration time <80msec [30]. Single studies used right atrial strain and RV free wall strain. Some studies were performed in specific patient populations, such as patients with diffuse pulmonary fibrosis, patients with systemic sclerosis, or liver transplant candidates, which could result in a lack of generalizability of results; however, this was a small minority of studies included in our meta-analysis. Overall, our results showed a pooled sensitivity of 85% and specificity of 71% for TTE compared to RHC. Additionally, it is important to note that both TTE and CMR can be useful to gauge response to therapy on regular follow-ups by demonstrating reversal of right ventricular dilatation and change in pulmonary vascular resistence.
The third noninvasive imaging modality used was CT scan, which, compared to MRI, is feasible to obtain and can also give information about the etiology of PH. The measure of diagnosis on CT is main pulmonary artery diameter (MPAD) with a cutoff > 29 mm with a reported diagnostic sensitivity of 87%, specificity of 89%, and PPV of 97% [5], [31]. The specificity of pH can reach up to 100% with MPAD > 29 in combination with segmental artery–to-bronchus diameter ratio of 1:1 in 3–4 lobes [32]. Other measures of diagnosis of PH on CT can be the main pulmonary artery to ascending aorta diameter ratio of ≥ 1 [5], [33], [34]. CT scan also helps to differentiate group-1 PH (secondary to lung disease and/or hypoxia) from group-3 PH (Idiopathic, drug/toxin-induced, connective tissue disease, and autoimmune cases) [15]. Shen et al. reported a sensitivity of 79% and specificity of 83% based on the pulmonary diameter and a sensitivity and specificity of 74% and 81% for TTE, respectively [5]. By contrast, our results included 27 studies comparing CT scan with RHC for diagnostic accuracy of PH. Our results were in concordance with Shen et al. study, with a sensitivity of 79% and specificity of 82% for both pulmonary artery diameter and PA:A ratio. We think this is a valuable conclusion as CT scans are readily available and done more frequently than ever, mostly for reasons other than PH [35]. In addition, CT scans can help in the diagnosis of concomitant lung parenchymal diseases associated with higher pulmonary vascular resistance (like bronchiectasis and interstitial lung disease). Furthermore, other non-numerical surrogate indicators of cor pulmonale can aid in the diagnosis of PH if seen by CT images (e.g: leftward septal deviation and thickening of right ventricular free wall). [14]
Overall, we believe that both TTE and CT have been falling out of favor in serially following patients diagnosed with PH due to operator dependency in the former and risk of radiation exposure in the latter. MRI, however, has been shown to be exceptionally reliable with good inter-study reproducibility in both healthy subjects and patients with heart failure.[36]
5. Limitations
Though the data supports MRI being the most sensitive and specific, further studies would need to be conducted to evaluate the cost relationship between the three imaging modalities. Issues such as the availability of the imaging modalities within different health systems around the world, as well as having the appropriate staff to read the imaging modality are required. A widespread variability in the measures and cutoffs of different parameters across the included studies limited our ability to measure its predictive effect. It is important to note that due to variable diagnostic criterias used by the included studies, our results might fail to identify a selected patient population who might have a normal or near-normal PAP. Some articles used the RV remodeling as a surrogate marker for PH, which could be misleading. Additionally, we could not take into account the pre-test probability of PH and could not include studies not reporting raw data of diagnostic accuracy.
6. Conclusions
MRI has the highest diagnostic accuracy among the noninvasive diagnostic modalities for the detection of pulmonary hypertension. Echocardiography is the most feasible test but has lower sensitivity and specificity.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Dr. Margot Boigon, Dr. David Smith and Dr. Richard Eisenstaedt for providing research opportunities and resources in the institute.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100568.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References:
- 1.Hatano S., Strasser T. Report on a WHO meeting; WHO, GENEVA: 1975. Primary pulmonary hypertension. [Google Scholar]
- 2.Chen Y1, Shlofmitz E, Khalid N, Bernardo NL, Ben-Dor I, Weintraub WS WR. Right Heart Catheterization-Related Complications: A Review of the Literature and Best Practices. Cardiol Rev. 2020;28(1):36–41. [DOI] [PubMed]
- 3.Simonneau G., Montani D., Celermajer D.S., Denton C.P., Gatzoulis M.A., Krowka M. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019 doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Alto M., Bossone E., Opotowsky A.R., Ghio S., Rudski L.G., Naeije R. Strengths and weaknesses of echocardiography for the diagnosis of pulmonary hypertension. International journal of cardiology. 2018;15(263):177–183. doi: 10.1016/j.ijcard.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 5.Shen Y., Wan C., Tian P., Wu Y., Li X., Yang T. CT-base pulmonary artery measurementin the detection of pulmonary hypertension. Med (United States) 2014 doi: 10.1097/MD.0000000000000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devaraj A., Wells A.U., Meister M.G., Loebinger M.R., Wilson R., Hansell D.M. Pulmonary hypertension in patients with bronchiectasis: Prognostic significance of CT signs. Am J Roentgenol. 2011 doi: 10.2214/AJR.10.5221. [DOI] [PubMed] [Google Scholar]
- 7.Rana B.S., Robinson S., Francis R., Toshner M., Swaans M.J., Agarwal S. Tricuspid regurgitation and the right ventricle in risk stratification and timing of intervention. Echo Research and Practice. 2019 doi: 10.1530/ERP-18-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nauser T.D., Stites S.W. Diagnosis and treatment of pulmonary hypertension. Am Fam Physician. 2001 [PubMed] [Google Scholar]
- 9.Geva T. MRI is the preferred method for evaluating right ventricular size and function in patients with congenital heart disease. Circ Cardiovasc Imaging. 2014 doi: 10.1161/CIRCIMAGING.113.000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogel-Claussen J., Shehata M.L., Lossnitzer D., Skrok J., Singh S., Boyce D. Increased right ventricular septomarginal trabeculation mass is a novel marker for pulmonary hypertension: Comparison with ventricular mass index and right ventricular mass. Invest Radiol. 2011 doi: 10.1097/RLI.0b013e31821b7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang N., Hu X., Liu C., Ali B., Guo X., Liu M. A Systematic Review of the Diagnostic Accuracy of Cardiovascular Magnetic Resonance for Pulmonary Hypertension. Canadian Journal of Cardiology. 2014 doi: 10.1016/j.cjca.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 12.Saba T.S., Foster J., Cockburn M., Cowan M., Peacock A.J. Ventricular mass index using magnetic resonance imaging accurately estimates pulmonary artery pressure. Eur Respir J. 2002 doi: 10.1183/09031936.02.00014602. [DOI] [PubMed] [Google Scholar]
- 13.Roeleveld R.J., Marcus T.J., Faes T.J.C., Gan T.J., Boonstra A., Postmus P.E. Interventricular septal configuration at MR imaging and pulmonary arterial pressure in pulmonary hypertension. Radiology. 2005 doi: 10.1148/radiol.2343040151. [DOI] [PubMed] [Google Scholar]
- 14.Kiely D.G., Levin D.L., Hassoun P.M., Ivy D., Jone P.N., Bwika J. Statement on imaging and pulmonary hypertension from the. Pulmonary Vascular Research Institute (PVRI). Pulm Circ. 2019 doi: 10.1177/2045894019841990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goerne H., Batra K., Rajiah P. Imaging of pulmonary hypertension: An update. Cardiovascular Diagnosis and Therapy. 2018 doi: 10.21037/cdt.2018.01.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swift A.J., Wild J.M., Nagle S.K., Roldán-Alzate A., François C.J., Fain S. Quantitative magnetic resonance imaging of pulmonary hypertension: A practical approach to the current state of the art. Journal of Thoracic Imaging. 2014 doi: 10.1097/RTI.0000000000000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forfia P.R., Fisher M.R., Mathai S.C., Housten-Harris T., Hemnes A.R., Borlaug B.A. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006 doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]
- 18.Hemnes A.R., Forfia P.R., Champion H.C. Assessment of pulmonary vasculature and right heart by invasive haemodynamics and echocardiography. International Journal of Clinical Practice. 2009 doi: 10.1111/j.1742-1241.2009.02110.x. [DOI] [PubMed] [Google Scholar]
- 19.Borges A.C., Knebel F., Eddicks S., Panda A., Schattke S., Witt C. Right Ventricular Function Assessed by Two-Dimensional Strain and Tissue Doppler Echocardiography in Patients With Pulmonary Arterial Hypertension and Effect of Vasodilator Therapy. Am J Cardiol. 2006 doi: 10.1016/j.amjcard.2006.02.060. [DOI] [PubMed] [Google Scholar]
- 20.Pirat B., McCulloch M.L., Zoghbi W.A. Evaluation of Global and Regional Right Ventricular Systolic Function in Patients With Pulmonary Hypertension Using a Novel Speckle Tracking Method. Am J Cardiol. 2006 doi: 10.1016/j.amjcard.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 21.Sciomer S., Magrì D., Badagliacca R. Non-invasive assessment of pulmonary hypertension: Doppler-echocardiography. Pulm Pharmacol Ther. 2007 doi: 10.1016/j.pupt.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Lang R.M., Bierig M., Devereux R.B., Flachskampf F.A., Foster E., Pellikka P.A. Recommendations for chamber quantification. European Journal of Echocardiography. 2006 doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Yock P.G., Popp R.L. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984 doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]
- 24.Hatle L., Angelsen B.A.J., Tromsdal A. Non-invasive estimation of pulmonary artery systolic pressure with Doppler ultrasound. Br Heart J. 1981 doi: 10.1136/hrt.45.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frost A., Badesch D., Gibbs J.S.R., Gopalan D., Khanna D., Manes A. Diagnosis of pulmonary hypertension. Eur Respir J. 2019 doi: 10.1183/13993003.01904-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Condliffe R., Radon M., Hurdman J., Davies C., Hill C., Akil M. CT pulmonary angiography combined with echocardiography in suspected systemic sclerosis-associated pulmonary arterial hypertension. Rheumatology (Oxford). 2011 doi: 10.1093/rheumatology/ker114. [DOI] [PubMed] [Google Scholar]
- 27.Rajaram S., Swift A.J., Capener D., Elliot C.A., Condliffe R., Davies C. Comparison of the diagnostic utility of cardiac magnetic resonance imaging, computed tomography, and echocardiography in assessment of suspected pulmonary arterial hypertension in patients with connective tissue disease. J Rheumatol. 2012 doi: 10.3899/jrheum.110987. [DOI] [PubMed] [Google Scholar]
- 28.Colin GC, Gerber BL, de Meester de Ravenstein C, Byl D, Dietz A, Kamga M, et al. Pulmonary hypertension due to left heart disease: diagnostic and prognostic value of CT in chronic systolic heart failure. Eur Radiol. 2018; [DOI] [PubMed]
- 29.Zhang R.F., Zhou L., Ma G.F., Shao F.C., Wu X.H., Ying K.J. Diagnostic value of transthoracic doppler echocardiography in pulmonary hypertension: A meta-analysis. American Journal of Hypertension. 2010 doi: 10.1038/ajh.2010.188. [DOI] [PubMed] [Google Scholar]
- 30.Opotowsky A.R., Ojeda J., Rogers F., Prasanna V., Clair M., Moko L. A simple echocardiographic prediction rule for hemodynamics in pulmonary hypertension. Circ Cardiovasc Imaging. 2012 doi: 10.1161/CIRCIMAGING.112.976654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan R.T., Kuzo R., Goodman L.R., Siegel R., Haasler G.B. Presberg KW. Utility of CT scan evaluation for predicting pulmonary hypertension in patients with parenchymal lung disease. Medical College of Wisconsin Lung Transplant Group. Chest. 1998 doi: 10.1378/chest.113.5.1250. [DOI] [PubMed] [Google Scholar]
- 32.Junqueira F.P., Lima C.M.A.O., Coutinho A.C., Parente D.B., Bittencourt L.K., Bessa L.G.P. Pulmonary arterial hypertension: An imaging review comparing MR pulmonary angiography and perfusion with multidetector CT angiography. British Journal of Radiology. 2012 doi: 10.1259/bjr/28150079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ng C.S., Wells A.U., Padley S.P.G. A CT sign of chronic pulmonary arterial hypertension: The ratio of main pulmonary artery to aortic diameter. J Thorac Imaging. 1999 doi: 10.1097/00005382-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Devaraj A., Hansell D.M. Computed tomography signs of pulmonary hypertension: old and new observations. Clinical Radiology. 2009 doi: 10.1016/j.crad.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Bellolio M.F., Heien H.C., Sangaralingham L.R., Jeffery M.M., Campbell R.L., Cabrera D. Increased computed tomography utilization in the Emergency Department and Its Association with Hospital Admission. West. J Emerg Med. 2017 doi: 10.5811/westjem.2017.5.34152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grothues F., Moon J.C., Bellenger N.G., Smith G.S., Klein H.U., Pennell D.J. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J. 2004;147(2):218–223. doi: 10.1016/j.ahj.2003.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.