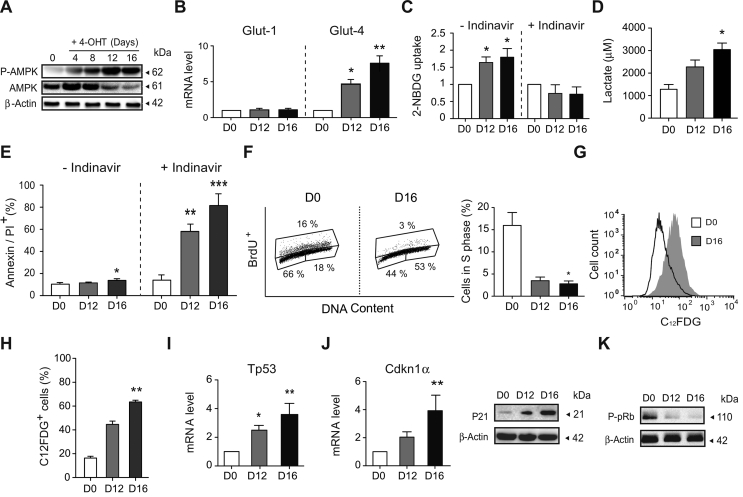
Figure 2.
Mitochondrial OXPHOS dysfunction was counterbalanced in AIF-/YMEFs by a shift towards anaerobic glycolysis and the development of a senescent phenotype. (A) Kinetic phosphorylation of AMPK visualized by immunoblot in left untreated (0) or 4-OHT-treated MEFs (4–16 days post-treatment). Equal loading was confirmed by β-Actin probing. This experiment was repeated three times with similar results. (B) Glut-1 and Glut-4 mRNA levels determined by quantitative RT-PCR in control (D0) and AIF-/Y MEFs (D12 and D16; n = 8). 18S mRNA expression was used to normalize data. Results are expressed as a ratio of mRNA expression relative to control (D0) cells (set at 1.0). (C) Glucose uptake measured by the assimilation of 2-NBDG in control (D0) and AIF-/Y MEFs (D12 and D16) untreated or pre-treated with indinavir (50 μM; n = 6). Results are expressed as a ratio relative to control (D0) cells (set at 1.0). (D) Lactate release, recorded in control (D0) and AIF-/Y MEFs (D12 and D16), was measured as described in the Methods section (n = 4). (E) Cytofluorometric assessment of cell death performed in control (D0) and AIF-/Y MEFs (D12 and D16) untreated or pre-treated with indinavir (50 μM) and labeled with AnnexinV and PI. The frequency of positive staining, which represents dying cells, was recorded and expressed as a plot (n = 6). (F) Flow cytometry cell cycle analysis performed in control (D0) and AIF-/Y MEFs (D12 and D16) by BrdU and PI (DNA content) co-labeling. Left, representative cytometric panels of control (D0) and AIF-/Y MEFs (D16). Right, the percent of cells in phase S was quantified and expressed as a plot (n = 4). (G) Cytometric evaluation of senescence in control (D0) and AIF-/Y MEFs (D12 and D16) using the β-galactosidase substrate C12FDG. Representative flow cytometric profiles of control (D0) and AIF-/Y MEFs (D16). (H) The percentage of C12FDG positive control (D0) and AIF-/Y MEFs (D12 and D16) measured as in (G) was calculated and graphed (n = 4). (I) Tp53 mRNA levels determined by qPCR in control (D0) and AIF-/Y MEFs (D12 and D16; n = 5). 18S mRNA expression was used to normalize data. Results are expressed as a ratio of mRNA expression relative to control (D0) cells (set at 1.0). (J) Left, Cdkn1α mRNA levels determined by qPCR in control (D0) and AIF-/Y MEFs (D12 and D16; n = 6). 18S mRNA expression was used to normalize data. Results are expressed as a ratio of mRNA expression relative to control (D0) cells (set at 1.0). Right, representative immunoblot of control (D0) and AIF-/Y MEFs (D12 and D16) revealing the cell cycle inhibitor P21. Equal loading was confirmed by β-Actin probing (n = 4 experiments with similar results). (K) Representative immunoblot of control (D0) and AIF-/Y MEFs (D12 and D16) revealing the decrease in pRb phosphorylation (P-pRb) associated with AIF loss. Equal loading was confirmed by β-Actin probing (n = 3 experiments with similar results). Statistical significance was calculated by Mann–Whitney (D, F, H, I) or student t (B, C, E, J) tests. Bars represent mean ± SEM.