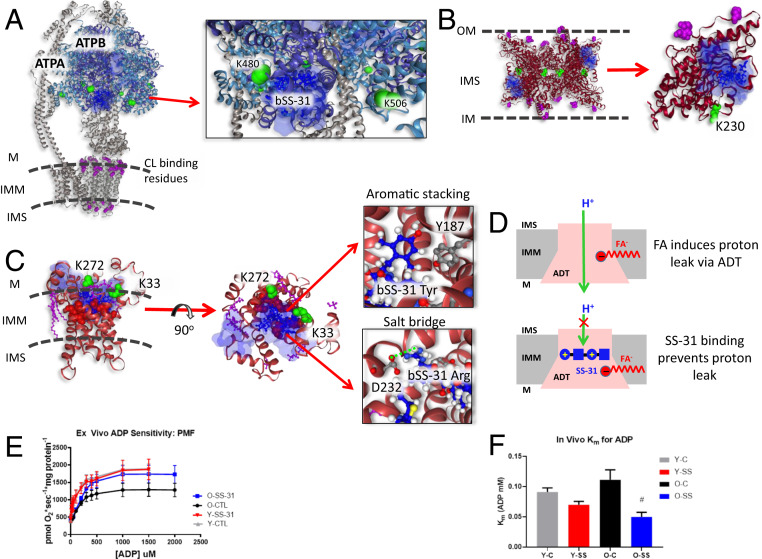
Fig. 4.
Structural and functional impact of bSS-31 interactions with ATP-producing enzymes. Distance constraints of 0 to 35 Å between the α-carbon of K3 of bSS-31 and the α-carbon of cross-linked lysine residues (green spheres) were used in molecular docking. The top 10 docking results are selected to show the ranges of bSS-31 positions in semitransparent blue surface view with the first docking position displayed in blue ball-and-stick view. CL and CL binding residues are shown in magenta ball-and-stick and space-filled representation, respectively. (A) Structure of bSS-31 interaction with ATP synthase with zoomed Inset. (B) A side view of octameric KCRS (PDB: 1crk) bridging outer and inner membranes of mitochondria is shown in cartoon representation at Left. Shown at Right is monomeric chain A in ribbon view with CL binding residues, K408, R418, and K419, in magenta space-filled balls, and cross-linked residue K230 in green spheres. (C) Structural representation of b-SS31 and ADT interaction in the BKA-locked m-state (PDB: 6gci). Cross-linked lysines K33 and K272 are shown in green spheres and CL is shown in magenta ball-and-stick view. The substrate binding sites, K23, R80, R280, Y187, G183, I184, and S228, are shown in red spheres. Interactive versions of the structures can be viewed at xlinkdb.gs.washington.edu/xlinkdb/BiotinylatedSS31_Bruce.php. Insets show detailed view of salt bridge interaction between D232 residue and Arg residue in bSS-31 and aromatic stacking between Y187 and dimethyl tyrosine in bSS-31. (D) Proposed model of FA-dependent proton leak via ADT (Top) (66), SS-31 binding of ADT possibly prevents proton leak with its two positively charged residues (Bottom). (E) SS-31 treatment increases ADP-stimulated respiration in mitochondria isolated from old mice. (F) SS-31 treatment decreases Km for ADP in vivo in old mice. #P < 0.05 using a paired t test compared to old control. Error bars represent the SEM.