Figure 4.
dPTPMT1 Depletion Results in Air Filling and Mitochondrial Defects in the Trachea
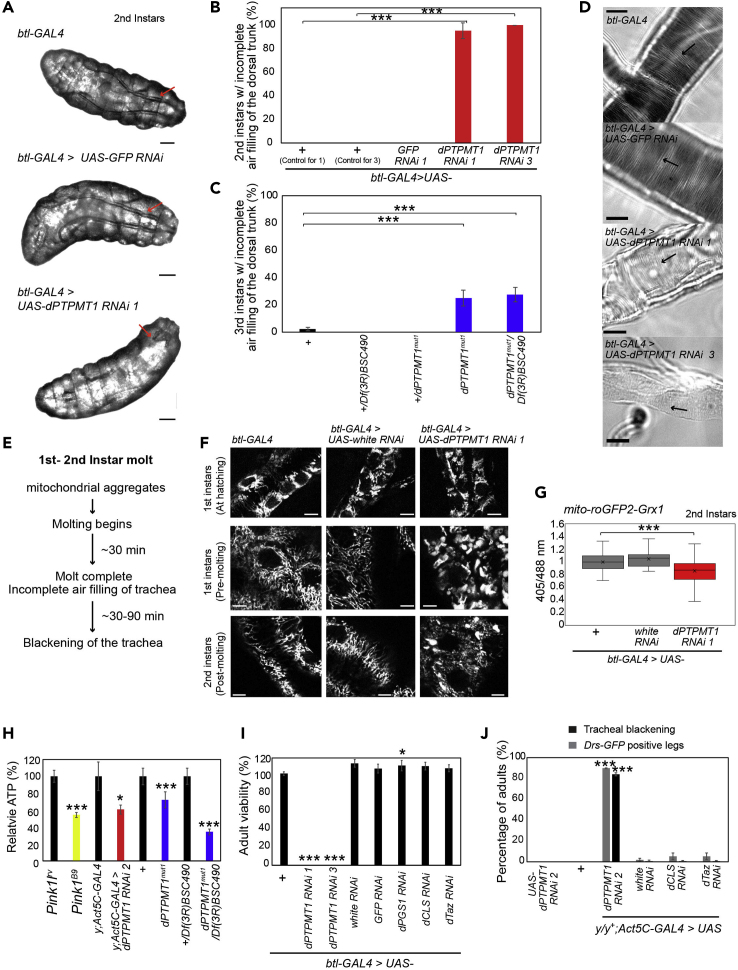
(A) Air-filling defects in second instars with tracheal-specific dPTPMT1 RNAi. Red arrows indicate trachea. In dPTPMT1 RNAi a portion of the posterior dorsal trunk shows air filling, but the remainder of the dorsal trunk is unfilled. Trachea filled with air appears darker than the adjacent tissues owing to differences in the refractive indices of liquid and air. Scale bars, 100 μm.
(B) Percentage of second instars with air-filling defects with tracheal-specific dPTPMT1 RNAi. n = 3 replicates, 6–12 for each repeat. Data are represented as mean ± SEM and ∗∗∗p < 0.001 by chi-square test.
(C) Percentage of dPTPMT1mut1 third instars showing tracheal air-filling defects. n = 3–6 replicates, 14–33 for each repeat. Data are represented as mean ± SEM and ∗∗∗p < 0.001 by chi-square test.
(D) Taenidial folds (arrows) in dissected trachea captured by light microscopy show no defects in tracheal-specific dPTPMT1 RNAi second instars. Note that these mutant tracheae are transparent because of the lack of air. The same results were seen in 8–22 larvae from two independent crosses. Scale bars, 100 μm.
(E) Timeline of events in tracheal-specific dPTPMT1 RNAi larvae undergoing first to second instar molt.
(F) Live imaging of mito-GFP within the dorsal trunk of the trachea of first instars at hatching, just prior to molting (“pre-molting”), and newly molted second instars (“post-molting”). The same results were seen in ten larvae from two independent crosses. Scale bars, 5 μm.
(G) Quantification of fluorescence ratios of mito-roGFP2-Grx1 expressed in trachea of second instars. n = 32–38 from three independent crosses. Boxes show 25th/75th percentiles, whiskers are the minimum and maximum values, and x is the median marker and ∗∗∗p < 0.001 by one-tailed Student's t test.
(H) Relative ATP levels, normalized to total protein. n = 7 independent experiments. Five-day-old Pink11RV (control) and Pink1B9 adult males (Park et al., 2006) were used as a positive control. Ubiquitous dPTPMT1 RNAi were adult males (0–2 days old) and dPTPMT1mut1 were third instars. Statistical significance is given relative to respective controls (black bar). Data are represented as mean ± SEM and ∗p < 0.05, ∗∗∗p < 0.001 by one-tailed Student's t test.
(I) Viability of adults with tracheal-specific RNAi of enzymes involved in CL biosynthesis raised at 25°C, 0- to 2-day-old adults. For simplicity only one background control is shown. Genotypic frequency is calculated for the genotype of interest and then normalized to its expected genotypic frequency. Statistical significance is given relative to respective controls. dPGS1 RNAi viability is significantly increased relative to its control. Data are represented as mean ± SEM and ∗p < 0.05, ∗∗∗p < 0.001 by chi-square test.
(J) Tracheal blackening and Drs-GFP expression in adult females (0–2 days old) with ubiquitous RNAi of CL biosynthesis enzymes. Females are heterozygous for y mutation (y/y+). Statistical significance is given relative to Act5C-GAL4>white RNAi. n = 3 replicates, 17–83 for each repeat. Data for Drs-GFP for controls and dPTPMT1 RNAi are the same as in Figure 3D.
Data are represented as mean ± SEM and ∗∗∗p < 0.001 by chi-square test. See also Figures S3 and S4.