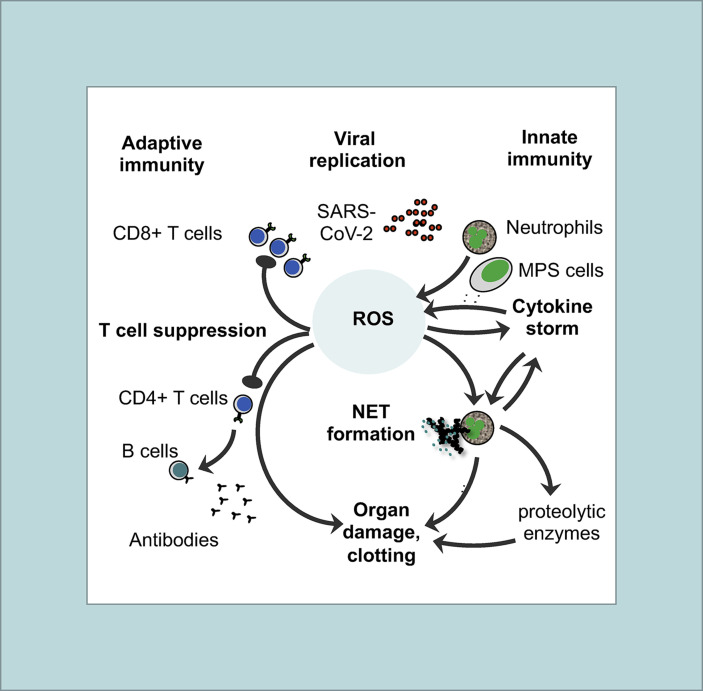
Fig. 1.
Basic features of the immunopathogenesis in COVID-19with central role of ROS: Both innate (right site) and adaptive immune responses (left site) are involved in immunopathogenesis of COVID-19. The pathogenic cascade starts with prolonged and extensive replication of SARS-CoV-2 in lung epithelial cells and in endothelial cells of the vessels due to viral evasion of the IFN-I/III response. As a consequence, neutrophils and MPS cells (e.g. macrophages, monocytes, and immature DCs) are massively recruited to the inflammatory tissue. Activated neutrophils and MPS cells produce large amounts of ROS thereby creating an imbalanced oxidative stress response. Oxidation of endogenous molecules (e.g. DNA and lipids) results in DAMPs that trigger pro-inflammatory cytokine secretion (cytokine storm) through TLR signaling thereby activating the redox-sensitive transcription factor NF-κB. ROS and TLR signaling also induce an overflow of NETs. There may be several positive feedback loops between cytokines (TNF-α, IL-1β) and ROS production as well as between cytokines (TNF-α, IL-1β) and NET formation. ROS, NETs and proteolytic enzymes released by activated neutrophils also contribute to organ damage and clotting in vessels. On the other side, imbalanced ROS production also suppresses the T cell response thereby contributing to lymphopenia in COVID-19. As a result, less activated antiviral CD8 T cells are available to kill virus-infected cells and to eliminate the virus. Moreover, CD4+ T cells are less efficient in helping B cells to produce neutralizing antibodies and establish long-term immunity.