Highlights
-
•
The dynamic changes in peripheral blood lymphocyte subsets were investigated in adult patients with COVID-19.
-
•
The levels of peripheral blood lymphocyte subsets (CD3+, CD4+, and CD8+) differed significantly between patients with non-severe and severe COVID-19.
-
•
The levels of peripheral blood lymphocyte subsets (CD3+, CD4+, and CD8+) differed significantly between patients who recovered and those who died of COVID-19.
-
•
Dynamic monitoring of human immune function is one of the indicators for evaluating the severity of disease and the prognosis of COVID-19 patients.
Keywords: Lymphocyte subsets, Cellular immunity, COVID-19, Severity, Prognosis
Abstract
Introduction
Coronavirus disease 2019 (COVID-19), caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread widely. The aim of this study was to investigate the dynamic changes in peripheral blood lymphocyte subsets in adult patients with COVID-19.
Methods
The electronic medical records were reviewed. Data including demographic characteristics, clinical manifestations, comorbidities, laboratory data, and radiological examinations of 435 hospitalized COVID-19 patients with a confirmed SARS-CoV-2 viral infection were extracted and analyzed retrospectively. Lymphocyte subset counts at each week after the onset of the illness were compared with those of the other weeks of illness and with those of control individuals.
Results
The various lymphocyte subsets (CD3+, CD4+, CD8+, CD19+, and CD16/56+) were below the normal ranges at 1 week after the onset of illness, reaching a nadir during the second week. They increased gradually during the third week and returned to normal levels in the fifth week, but were still lower than those of the healthy controls. The CD3+, CD4+, and CD8+ counts were significantly lower in patients with severe disease compared to those with non-severe disease, and in patients who died compared to those who recovered.
Discussion
This research indicates that the levels of peripheral blood lymphocyte subsets (CD3+, CD4+, and CD8+) are associated with disease progression and severity, and with the prognosis in patients with COVID-19. Dynamic monitoring of human immune function is one of the indicators for evaluating the severity of disease and the prognosis of COVID-19 patients, and is useful for formulating appropriate treatment strategies.
Introduction
Cases of pneumonia of unknown etiology emerged in Wuhan, Hubei Province, China in December 2019. Studies have shown that the disease is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the disease was recently named coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO) (Huang et al., 2020, Chen et al., 2020). This new coronavirus has a long incubation period and is highly contagious (World Health Organization, 2020). Patients with COVID-19 have symptoms such as fever, a dry cough, and fatigue in the early stage, and may later develop acute respiratory distress syndrome (ARDS), respiratory failure, shock, and multiple organ failure, which may be fatal (Chen et al., 2020, Lu et al., 2020). According to the government, there were 82 919 confirmed cases and 4633 deaths reported in China by May 12, 2020 (NHC, 2020). Through a series of preventive control and medical treatment measures, the epidemic situation in China has been controlled to a certain extent, but cases of morbidity and death outside China have exceeded those of China and are on the rise. Therefore, COVID-19 is a major global health issue with high morbidity and mortality.
Lymphocytes are the main immune cells that eliminate the virus. In conventional viral infections, the proportion of lymphocytes in peripheral blood usually increases. However, peripheral lymphocytopenia was found in severe pandemic H1N1 influenza A and severe acute respiratory syndrome (SARS) (Cunha et al., 2009, He et al., 2005). T lymphocytes play a key role in adaptive immunity against influenza A virus infection and can reduce the severity of the symptoms during infection. A study found that the total number of lymphocytes, CD3+ T-cells, CD4+ T-cells, and CD8+ T-cells decreased significantly in patients with influenza A/H1N1 in the acute phase (Chen et al., 2018). Peripheral lymphocytopenia has also been found in patients with severe COVID-19 (Huang et al., 2020, Chen et al., 2020, Lu et al., 2020, Zhang et al., 2020). The seventh edition of the COVID-19 Diagnosis and Treatment Scheme issued by the National Health Commission of China (NHC China, 2020) pointed out that the progressive decline in peripheral blood lymphocyte count and the continuous decline in CD4+ T-cells and CD8+ T-cells were indicators of a deterioration in the patient's condition. Although many clinical studies have reported peripheral lymphocytopenia in patients with COVID-19 in laboratory tests, the dynamic changes in peripheral blood lymphocyte subsets in patients with COVID-19 have rarely been reported. The aim of this retrospective study was to determine the dynamic changes in peripheral blood lymphocyte subsets in patients with COVID-19.
Materials and methods
Research objects and data collection
Between January 31, 2020 and March 21, 2020, 435 patients with confirmed COVID-19 based on the diagnostic guidelines of the Chinese Ministry of Health, were admitted to the East Hospital of Renmin Hospital, Wuhan University. The East Hospital of Renmin Hospital is one of the designated hospitals for the admission of patients with COVID-19. The clinical data of these patients were analyzed retrospectively.
COVID-19 was defined according to the diagnostic and treatment guidelines for SARS-CoV-2 issued by the Chinese National Health Committee (version 7). In addition, patients in the non-severe group (NS) fulfilled at least one of the following criteria: dyspnea (respiratory rate ≥30 min–1), hypoxemia (SpO2 ≤93% whilst on oxygen at rest), oxygenation index ≤300 mmHg (arterial partial pressure of oxygen/inspired oxygen fraction, PaO2/FiO2), and chest imaging showing obvious progression of lesions over 95% within 48 hours. Patients in the severe group (S) had at least one of the following features: respiratory failure requiring mechanical ventilation, shock, and multiple organ dysfunction syndrome (MODS). The patients were divided retrospectively into those who recovered (415 cases) and those who died from COVID-19 (20 cases), according to the clinical prognosis of the disease.
Demographic information, clinical characteristics (including medical history, exposure history, comorbidities, surgery history, onset of initial symptoms and signs), laboratory findings, chest imaging findings, and clinical outcomes were retrieved from the information system of Renmin Hospital of Wuhan University. All clinical data were collected independently by two investigators. Day 1 of illness was defined as the day of fever onset. Control lymphocyte counts were obtained from 61 healthy individuals who were not affected by SARS-CoV-2; 33 were male and 28 were female. The changes in lymphocyte subsets were measured during 6 weeks of disease development. This study was approved by the Ethics Committee of Renmin Hospital, Wuhan University (No. WDRY2020-K144).
Detection of lymphocyte subsets
Peripheral blood samples were processed within 2 h of collection by flow cytometry to analyze the lymphocyte subsets. The lymphocyte subsets were identified using the following monoclonal antibodies: anti-CD3-FITC, anti-CD8-PE, anti-CD4-APC, anti-CD16/56-PE, and anti-CD19-APC. The cell suspension was incubated at room temperature in the dark for 30 min. Red blood cells were removed using 500 μl of lysis buffer at room temperature in the dark for 10 min. Finally, the cells were analyzed using FACS Canto flow cytometry and CellQuest software (BD BioScience). The counts of CD3+, CD4+, and CD8+ T lymphocytes, CD19+ cells, and CD16/56+ cells were obtained.
Statistical analysis
All data were inputted into an Excel database. The statistical analysis was performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Percentages and frequency rates were used to describe categorical variables; the mean, median, interquartile range (IQR), and standard deviation (SD) were used to describe continuous variables. The t-test or Mann–Whitney test was used to compare the means of continuous variables. The Chi-square test or Fisher's exact test was used to compare proportions of categorical variables. A p-value < 0.05 was considered statistically significant.
Results
The average age of the 435 patients with COVID-19 was 58 ± 25 years; 236 (54.2%) were over 60 years of age. There were 229 (52.6%) male patients and 206 (47.4%) female patients. Chest imaging revealed 374 patients with bilateral lung infections. Overall, 211 patients had comorbidities, including hypertension (121 cases), diabetes (80 cases), coronary heart disease (32 cases), and chronic airway disease (15 cases). Four hundred and four (92.9%) patients received antibiotics, 412 (94.7%) patients received antivirals, and 125 (28.7%) patients received systemic corticosteroids. Intravenous immunoglobulin use differed significantly between the non-severe and severe groups. The median time from illness onset to hospital admission was 10.0 days (IQR 8.0–14.0 days) and the median time to discharge or death was 24.0 days (IQR 20.0–31.0 days). The median time from illness onset to sepsis and ARDS was 12.0 days (IQR 9.0–15.0 days) and 14.0 days (IQR 10.0–17.0 days), respectively. Non-invasive mechanical ventilation and invasive mechanical ventilation use differed significantly between the non-severe and severe groups (Table 1 ). The patients with reverse transcriptase PCR (RT-PCR) results for SARS-CoV-2 showed negative results at 6 weeks, but still needed oxygen or treatment for complications.
Table 1.
Demographic and clinical characteristics of the patients, and treatments used.
| Variable | Total (n = 435) | Non-severe (n = 368) | Severe (n = 67) | p-Value |
|---|---|---|---|---|
| Age, years, median (IQR) | 58.0 (49.0–69.0) | 55.0 (45.0–63.0) | 65.0 (59.0–70.0) | <0.001 |
| Sex, n (%) | 0.73 | |||
| Male | 229 (52.6%) | 190 (51.6%) | 39 (58.2%) | |
| Female | 206 (47.4%) | 178 (48.4%) | 28 (41.8%) | |
| Comorbidities, n (%) | ||||
| Hypertension | 121 (27.8%) | 85 (23.1%) | 36 (53.7%) | <0.001 |
| Diabetes | 80 (18.4%) | 45 (12.2%) | 35 (52.2%) | <0.001 |
| Cardiovascular disease | 32 (8.7%) | 14 (3.8%) | 18 (26.9%) | <0.001 |
| Chronic airway disease | 15 (3.4%) | 9 (2.4%) | 6 (8.9%) | 0.02 |
| Cough | 265 (60.9%) | 226 (61.4%) | 39 (58.2%) | 0.72 |
| Chest congestion | 100 (23.0%) | 88 (23.9%) | 12 (17.9%) | 0.36 |
| Myalgia | 39 (9.0%) | 29 (7.9%) | 10 (14.9%) | 0.10 |
| Fatigue | 67 (15.4%) | 56 (15.2%) | 11 (16.4%) | 0.94 |
| Diarrhea | 21 (4.8%) | 15 (4%) | 6 (8.9%) | 0.16 |
| Dyspnea | 33(7.8%) | 28 | 5 | 0.83 |
| Radiological characteristics, n (%) | <0.001 | |||
| Unilateral | 61 (14%) | 61 (16.6%) | 0 | |
| Bilateral | 374 (86%) | 307 (83.4%) | 67 (100%) | |
| Treatments, n (%) | ||||
| Antibiotics | 404 (92.9%) | 339 (92.1%) | 65 (97.0%) | 0.24 |
| Antiviral treatment | 412 (94.7%) | 346 (94.0%) | 66 (98.5) | 0.22 |
| Corticosteroids | 125 (28.7%) | 104 (28.2%) | 21 (31.3%) | 0.71 |
| Intravenous immunoglobulin | 186 (42.8%) | 146 (39.7%) | 40 (59.7) | <0.01 |
| High-flow nasal cannula oxygen therapy | 177 (40.6%) | 157 (36.1%) | 20 (29.8%) | 0.07 |
| Non-invasive mechanical ventilation | 130 (29.9%) | 82 (22.3%) | 48 (71.6%) | <0.001 |
| Invasive mechanical ventilation | 71 (16.3%) | 23 (6.3%) | 48 (71.6%) | <0.001 |
| Time from illness onset to hospital admission, days, median (IQR) | 10.0 (8.0–14.0) | 9.0 (7.0–13.0) | 10.0 (8.0–15.0) | 0.57 |
| Time from illness onset to sepsis, days, median (IQR) | 12.0 (9.0–15.0) | 13.0 (9.0–16.0) | 12.0 (9.0–14.0) | 0.32 |
| Time from illness onset to ARDS, days, median (IQR) | 14.0 (10.0–17.0) | 15.0 (10.0–18.0) | 14.0 (10.0–17.0) | 0.45 |
| Time from illness onset to death or discharge, days, median (IQR) | 24.0 (20.0–31.0) | 23.0 (19.0–30.0) | 25.0 (21.0–32.0) | 0.13 |
IQR, interquartile range; ARDS, acute respiratory distress syndrome. p-Values were calculated by Mann–Whitney U-test, Chi-square test, or Fisher's exact test, as appropriate.
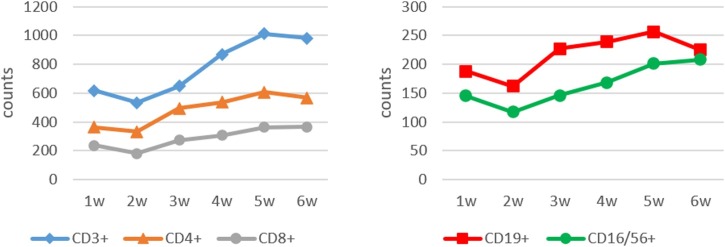
The lymphocyte subset counts in 1545 samples collected from the 435 patients with COVID-19 and 61 controls were analyzed (Table 2 ). The various lymphocyte subsets (CD3+, CD4+, CD8+, CD19+, and CD16/56+) were below the normal ranges in the first week after the onset of illness, reaching a nadir during the second week; they then increased gradually in the third week and returned to normal levels in the fifth week, but were still lower than those of the healthy controls. There were significant decreases in the CD3+, CD4+, and CD8+ counts over each of the 6 weeks of COVID-19 illness when compared to healthy controls. The CD16/56+ count did not change significantly between weeks 3 and 4, or between weeks 5 and 6. The CD19+ count did not change significantly except between weeks 2 and 3 (Table 2, Figure 1).
Table 2.
Changes in lymphocyte subset counts in COVID-19 patients compared with control individuals over 6 weeks of illness
| Time | Sample | Lymphocyte count (×106/L) |
||||
|---|---|---|---|---|---|---|
| CD3+ | CD4+ | CD8+ | CD19+ | CD16/CD56+ | ||
| 1 week | 82 | 617 ± 3251,3 | 361 ± 2051,4 | 236 ± 1421,3 | 188 ± 1061,4 | 145 ± 1011,3 |
| 2 weeks | 361 | 533 ± 3201,5 | 332 ± 2101,5 | 180 ± 1451,5 | 162 ± 1251,5 | 117 ± 901,5 |
| 3 weeks | 387 | 674 ± 3471,7 | 495 ± 2751,7 | 273 ± 1721,8 | 227 ± 1601,8 | 146 ± 1021,6 |
| 4 weeks | 303 | 869 ± 4261,9 | 536 ± 2801,9 | 306 ± 1931,9 | 239 ± 1602,10 | 168 ± 1322,9 |
| 5 weeks | 221 | 1010 ± 4601,12 | 607 ± 2931,12 | 362 ± 2241,12 | 256 ± 1242,12 | 201 ± 1422,12 |
| 6 weeks | 130 | 981 ± 4321,13 | 567 ± 2551,13 | 365 ± 2011,13 | 225 ± 1421,14 | 208 ± 1322,13 |
| Controls | 61 | 1348 ± 376 | 739 ± 268 | 566 ± 266 | 269 ± 106 | 197 ± 138 |
Counts expressed as the number of cells × 106/L ± 1 standard deviation. The lymphocyte subset counts were compared between patients with COVID-19 and normal individuals: 1p < 0.05, 2p > 0.05. The lymphocyte subset counts in week 2 of patients with COVID-19 compared to week 1 of illness: 3p < 0.05, 4p > 0.05. The lymphocyte subset counts in week 3 of patients with COVID-19 compared to week 2 of illness: 5p < 0.01, 6p > 0.05. The lymphocyte subset counts in week 4 of patients with COVID-19 compared to week 3 of illness: 7p < 0.05, 8p > 0.05. The lymphocyte subset counts in week 5 of patients with COVID-19 compared to week 4 of illness: 9p < 0.01, 10p > 0.05. The lymphocyte subset counts in week 6 of patients with COVID-19 compared to week 5 of illness: 11p < 0.05, 12p > 0.05. The lymphocyte subset counts in week 6 of patients with COVID-19 compared to week 1 of illness: 13p < 0.01, 14p > 0.05.
Figure 1.
Dynamic change in CD3+, CD4+, CD8+, CD19+, and CD16/56+ lymphocyte subsets (expressed as the mean number of cells ×106/L) measured over 6 weeks of illness in patients with COVID-19.
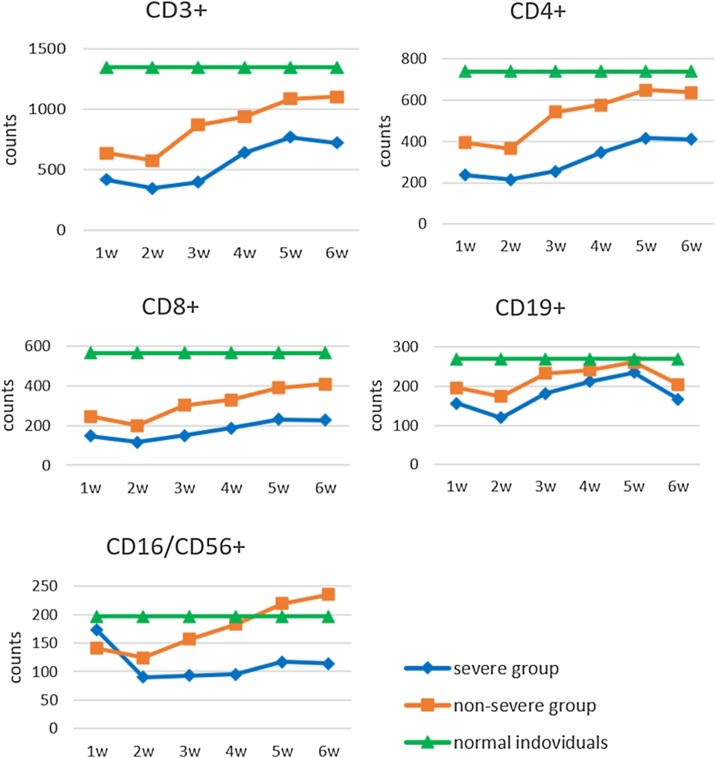
The lymphocyte subset counts were compared between the severe group (272 samples from 67 patients with COVID-19) and the non-severe group (1212 samples from 368 patients with COVID-19) at various time points (within 6 weeks), as shown in Table 3. The CD3+, CD4+, CD8+, CD19+, and CD16/56+ counts decreased significantly in the patients in the severe group and non-severe group compared to the healthy controls, but the CD19+ counts did not change significantly. The CD3+, CD4+, CD8+, and CD19+ counts were significantly lower in those with severe disease compared to those with non-severe disease, although the CD19+ counts did not change significantly. The CD16/56+ count was higher in those with severe disease in the first week, but the difference was not statistically significant. However, the CD16/56+ count decreased significantly in those with severe disease compared to those with non-severe disease in the other time periods (Figure 2).
Table 3.
Lymphocyte subset counts in severe and non-severe COVID-19 cases
| Time | Sample | Lymphocyte count (×106/L) |
||||
|---|---|---|---|---|---|---|
| CD3+ | CD4+ | CD8+ | CD19+ | CD16/CD56+ | ||
| 1 week | Non-severe (n = 56) | 628 ± 3501,5 | 374 ± 2261,5 | 235 ± 1431,5 | 193 ± 1622,5 | 141 ± 812,5 |
| Severe (n = 26) | 419 ± 2653 | 217 ± 1293 | 147 ± 1193 | 156 ± 663 | 173 ± 1194 | |
| 2 weeks | Non-severe (n = 301) | 576 ± 3211,5 | 365 ± 2131,5 | 200 ± 1451,5 | 174 ± 1102,5 | 124 ± 971,5 |
| Severe (n = 60) | 348 ± 2383 | 204 ± 1623 | 116 ± 1033 | 119 ± 813 | 90 ± 853 | |
| 3 weeks | Non-severe (n = 326) | 873 ± 4111,5 | 541 ± 2761,5 | 302 ± 1711,5 | 232 ± 1632,5 | 157 ± 1101,5 |
| Severe (n = 61) | 397 ± 3053 | 255 ± 1753 | 149 ± 1363 | 181 ± 1423 | 93 ± 873 | |
| 4 weeks | Non-severe (n = 250) | 937 ± 4361,5 | 576 ± 2831,5 | 330 ± 1991,5 | 241 ± 1682,6 | 183 ± 1311,6 |
| Severe (n = 53) | 640 ± 3753 | 345 ± 2273 | 188 ± 1853 | 212 ± 1533 | 95 ± 853 | |
| 5 weeks | Non-severe (n = 179) | 1086 ± 4241,5 | 648 ± 2741,5 | 391 ± 2001,5 | 261 ± 1882,6 | 219 ± 1451,6 |
| Severe (n = 42) | 771 ± 3693 | 416 ± 2423 | 231 ± 1533 | 235 ± 2154 | 117 ± 953 | |
| 6 weeks | Non-severe (n = 100) | 1003 ± 4061,5 | 637 ± 2361,5 | 408 ± 1991,5 | 204 ± 1572,6 | 235 ± 1531,6 |
| Severe (n = 30) | 722 ± 3003 | 410 ± 1783 | 227 ± 1463 | 167 ± 1463 | 114 ± 993 | |
| Controls | Control (n = 61) | 1348 ± 376 | 739 ± 268 | 566 ± 266 | 269 ± 106 | 197 ± 138 |
Counts expressed as the number of cells × 106/L ± 1 standard deviation. Comparison of lymphocyte subset counts between the severe and non-severe cases with COVID-19 at each week (1–6) of illness: 1p < 0.05, 2p > 0.05. Comparison of lymphocyte subset counts between the severe cases with COVID-19 and the control group at each week (1–6) of illness: 3p < 0.05, 4p > 0.05. Comparison of lymphocyte counts between non-severe cases with COVID-19 and the control group at each week (1–6) of illness: 5p < 0.05, 6p > 0.05.
Figure 2.
Dynamic changes in lymphocyte subsets (expressed as the mean number of cells ×106/L) measured over the first 6 weeks of illness in patients with non-severe and severe COVID-19, and in healthy controls.
The peripheral blood lymphocyte subsets were also compared between the patients who recovered (1412 samples from 415 patients with COVID-19) and the patients who died (72 samples from 20 patients with COVID-19) in each period (within 6 weeks), as shown in Table 4. Patients who died had significantly lower CD3+, CD4+, and CD8+ counts than those who recovered. The difference in CD19+ counts was not statistically significant. The CD16/56+ count was higher in those who died in the first week, but the difference was not statistically significant. However, the CD16/56+ count was significantly lower in the patients who died compared to those who recovered in the other time periods.
Table 4.
Lymphocyte subset counts in patients who recovered and those who died of COVID-19
| Time | Samples | Lymphocyte count (×106/L) |
||||
|---|---|---|---|---|---|---|
| CD3+ | CD4+ | CD8+ | CD19+ | CD16/CD56+ | ||
| 1 week | Recovered (n = 76) | 584 ± 2171 | 353 ± 1981 | 218 ± 1521 | 187 ± 1052 | 149 ± 942 |
| Died (n = 6) | 368 ± 170 | 229 ± 101 | 162 ± 92 | 141 ± 95 | 175 ± 102 | |
| 2 weeks | Recovered (n = 343) | 550 ± 2371 | 346 ± 1591 | 190 ± 1051 | 167 ± 1022 | 120 ± 861 |
| Died (n = 18) | 305 ± 218 | 209 ± 172 | 106 ± 100 | 122 ± 87 | 85 ± 84 | |
| 3 weeks | Recovered (n = 370) | 822 ± 3291 | 509 ± 1861 | 286 ± 1491 | 228 ± 1302 | 150 ± 941 |
| Died (n = 17) | 275 ± 183 | 202 ± 131 | 97 ± p61 | 155 ± 104 | 60 ± 38 | |
| 4 weeks | Recovered (n = 288) | 910 ± 3821 | 549 ± 2301 | 315 ± 1981 | 239 ± 1982 | 174 ± 1031 |
| Died (n = 15) | 403 ± 328 | 278 ± 212 | 120 ± 105 | 175 ± 114 | 43 ± 41 | |
| 5 weeks | Recovered (n = 212) | 1045 ± 4001 | 614 ± 2541 | 368 ± 1641 | 259 ± 1722 | 205 ± 991 |
| Died (n = 9) | 581 ± 218 | 359 ± 136 | 185 ± 88 | 179 ± 147 | 53 ± 28 | |
| 6 weeks | Recovered (n = 123) | 1043 ± 3761 | 601 ± 1961 | 379 ± 1441 | 199 ± 1022 | 215 ± 1041 |
| Died (n = 7) | 526 ± 337 | 296 ± 206 | 137 ± 121 | 167 ± 132 | 58 ± 49 | |
Counts expressed as the number of cells × 106/L ± 1 standard deviation. Comparison of lymphocyte subset counts between the patients who died and those who recovered at each week (1–6) of illness: 1p < 0.05, 2p > 0.05.
Discussion
Lymphocytosis has been found in acute (severe measles (Ryon et al., 2002), SARS-COV (Peiris et al., 2003), Ebola virus (Auwaerter et al., 1999)) and chronic (HIV infection (Wong et al., 2015)) viral infections, as well as in non-communicable diseases (systemic lupus erythematosus (Boag et al., 2015), cerebral hemorrhage (Giede-Jeppe et al., 2016)). However, these diseases have their own clinical characteristics, so the lymphocytopenia that occurs in the above diseases can be easily distinguished from the lymphopenia in COVID-19 infection. Recently, many clinical studies have reported peripheral blood lymphocytopenia in laboratory tests of patients with COVID-19, but the studies have not reported dynamic changes in peripheral blood lymphocyte subsets of patients with COVID-19.
Cellular immunity and humoral immunity are important defense systems of the human body, and T lymphocyte subsets are an important part of the immune cell population and also an important indicator for detecting cellular immune functions. There are two important subsets of T lymphocytes: CD3+ CD4+ helper T lymphocytes and CD3+ CD8+ cytotoxic T lymphocytes. They play an important role in regulating cellular and humoral immunity. The proportions of T lymphocyte cells and their subsets maintain a dynamic homeostasis in the body, acting as agonists or inhibitors to maintain the body's normal immune regulatory functions (Liu et al., 2017, Li et al., 2014). CD3+ is an important marker for identifying T-cells and is involved in specific antigen recognition. CD4+ cells can enhance the immune response and exert anti-tumor cell effects and other effects mainly by secreting cytokines. CD8+ cells can suppress the activation of the immune response and affect the function of hematopoietic stem cells by inducing cytotoxic effects or secreting hematopoietic inhibitory factors. In this study, we evaluated the dynamic changes in peripheral lymphocyte subsets over the course of disease (within 6 weeks) in patients with COVID-19.
The changes in peripheral blood lymphocyte subsets were measured in 435 patients with COVID-19 for at least 6 weeks. After analysis, it was found that the CD3+ and CD4+ subsets decreased significantly during the first week of the disease and were below the normal range, while the CD8+ subset was still within the normal clinical reference range. In the second week, the CD3+, CD4+, and CD8+ counts reached their lowest points, which were below the normal clinical reference value; they then increased gradually during the third week and returned to normal levels in the fifth week. Compared with the healthy control group, the lymphocyte counts of patients with severe and non-severe disease were significantly lower, suggesting that T-cell levels have a downward trend in severe COVID-19 patients with fever as the first symptom. The reaction is related, resulting in a decline in the body's immune function. The data also showed that the CD3+, CD4+, and CD8+ counts were lower in more severely ill patients and those who died, which may be related to the systemic inflammatory response, leading to a decline in immune function. That is to say, the lower the immune function, the more serious it is. This also suggests that T lymphocyte subsets (CD3+, CD4+, and CD8+) could be used as an indicator of the severity and prognosis of patients with COVID-19.
Natural killer (NK) cells are an important component of the innate immune system. They are the first line of defense against the invasion of exotic microorganisms. Before specific immune activation, innate immunity has been activated and plays an important role in resisting bacterial and viral infections (Cao et al., 2012). CD16/56+ NK cells started to decrease in the first week, reaching their lowest level in the second week, and had returned to normal by week 5. The data showed that the CD16/56+ counts were higher in more severely ill patients in the first week, while the CD16/56+ counts in the severely ill patients were lower than those in the non-severely ill patients in the other time periods. Similar findings were found in the comparison between the patients who recovered and those who died. The above results indicate that the innate immune function decreased gradually in the first week following symptom onset, the immune function decreased more rapidly in the severely ill patients and those who died, and the weakest innate immune function was in the second week. This study found that the level of T lymphocytes reached the lowest point in the second week, when the body's cellular immune function was also the weakest and the resistance was the worst. At this time, there may be further aggravation of viral or bacterial infections, which may be a pivotal point in the disease course. However, this needs further confirmation.
The reason for the decrease in peripheral blood lymphocytes may be due to the redistribution and migration of lymphocytes to the respiratory system to resist the virus, and lymphocyte transport mediated by cytokines also plays a role in this process. This study found that lymphocytes gradually decreased in the first and second weeks after the onset of illness, but from the third week, the lymphocyte counts gradually increased. It is possible that lymphocytes from the early circulation entered the inflammatory lung tissue, and lymphocytes from the inflammatory lung tissue re-entered the circulation in the later period. Liu et al. (He et al., 2020) reported that the T-cell counts in those who died remained at a low level. This study showed an increase in the T-cell counts during weeks 4 and 5 in the group of patients who died, but the T-cell counts had not returned to normal. This change did not alter the outcomes of these patients, suggesting that the recovery of immune function in such patients is slow. During treatment, proper use of corticosteroids should be paid attention to, as well as avoiding complications leading to death, such as secondary infections, cerebral hemorrhage, organ failure, etc. A recent autopsy report (Xu et al., 2020) showed that the lungs of patients with COVID-19 who died had increased levels of lymphocytic infiltrates. The above results support the hypothesis of lymphocyte redistribution. The reason for the lymphocyte decrease may also be that the virus directly infects and destroys lymphocytes, or it may be related to the apoptosis of lymphocytes induced by viral infection (Sun et al., 2017, Bahleda et al., 2016).
There were some limitations to this study. This was a retrospective study and the sample size was not sufficiently large. Furthermore, the number of patients in each time period was not consistent and some clinical data were lacking. There are several reasons for this. First, patients in the early stage following symptom onset were not hospitalized. Second, clinicians did not pay attention to cellular immunity. Third, patient discharge from the hospital or death affected the collection of samples. Further research is needed to overcome these limitations. Nevertheless, the observation time of this study was long and the sample size at the key time points was large. In addition, the trend in variation of cellular immune function during the treatment of diseases is not affected by the factors reported above. Thus the study provides useful evidence and highlights important results on this topic.
In summary, this study indicates that the levels of peripheral blood lymphocyte subsets (CD3+, CD4+, and CD8+) are associated with disease progression and severity, and the prognosis in patients with COVID-19. Dynamic monitoring of human immune function is one of the indicators for determining the severity of disease and prognosis for COVID-19 patients, and it is useful in formulating appropriate treatment strategies.
Conflict of interest
None declared.
References
- Auwaerter P.G., Rota P.A., Elkins W.R., Adams R.J., Delozier T., Shi Y. Measles virus infection in rhesus macaques: altered immune responses and comparison of the virulence of six different virus strains. J Infect Dis. 1999;180:950–958. doi: 10.1086/314993. [DOI] [PubMed] [Google Scholar]
- Bahleda R., Baker J., Massard C., Gadgeel S.M., Rogers J.E., Izzedine H. Phase I doseescalation and pharmacokinetic study of intravenous aflibercept in combination with docetaxel, cisplatin, and 5-fluorouracil in patients with advanced solid malignancies. Oncology. 2016;90(1):10–20. doi: 10.1159/000440958. [DOI] [PubMed] [Google Scholar]
- Boag S.E., Das R., Shmeleva E.V., Bagnall A., Egred M., Howard N. T lymphocytes and fractalkine contribute to myocardial ischemia/reperfusion injury in patients. J Clin Invest. 2015;125(8):3063–3076. doi: 10.1172/JCI80055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M.L., Tang Y.B., Wei S.J., Zhong J.B. Analysis of peripheral blood T cells and B cells and NK lymphocyte subsets in HIV-l-infected individuals. Inter J Epidemiol Infect Dis. 2012;39:25–28. [Google Scholar]
- Chen X., Liu S., Goraya M.U., Huang S., Chen J. Host immune response to influenza A virus infection. Front Immunol. 2018;9:320. doi: 10.3389/fimmu.2018.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha B.A., Pherez F.M., Schoch P. Diagnostic importance of relative lymphopenia as a marker of swine influenza (H1N1) in adults. Clin Infect Dis. 2009;49(9):1454–1456. doi: 10.1086/644496. [DOI] [PubMed] [Google Scholar]
- Giede-Jeppe A., Bobinger T., Gerner S.T., Mad\v{z}ar D., Sembill J., Lücking H. Lymphocytopenia is an independent predictor of unfavorable functional outcome in spontaneous intracerebral hemorrhage. Stroke. 2016;47(5):1239–1246. doi: 10.1161/STROKEAHA.116.013003. [DOI] [PubMed] [Google Scholar]
- He Z.P., Zhao C.H., Dong Q.M., Zhuang H., Song S., Peng G. Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. Int J Infect Dis. 2005;9:323–330. doi: 10.1016/j.ijid.2004.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He R., LU Z., Zhang L., Fan T., Xiong R., Shen X. The clinical course and its correlated immune status in COVID-19 pneumonia. J Clin Virol. 2020;127:104361. doi: 10.1016/j.jcv.2020.104361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Cai C., Feng J., Li X., Wang Y., Yang J. Peripheral T lymphocyte subset imbalances in children with enterovirus 71-induced hand, foot and mouth disease. Virus Res. 2014;180:84–91. doi: 10.1016/j.virusres.2013.11.021. [DOI] [PubMed] [Google Scholar]
- Liu J., Wang L., Feng Z., Geng D., Sun Y., Yuan G. Dynamic changes of laboratory parameters and peripheral blood lymphocyte subsets in severe fever with thrombocytopenia syndrome patients. Int J Infect Dis. 2017;58:45–51. doi: 10.1016/j.ijid.2017.02.017. [DOI] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020 doi: 10.1016/s0140-6736(20)30251-8. pii: S0140-6736(20)30251-8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- National health commission of the People's Republic of China. http://www.nhc.gov.cn/xcs/yqfkdt/202005/e6a7ad0d28294497be078c9fd588ea76.shtml. [DOI] [PMC free article] [PubMed]
- National Health Commission of the People,s Republic of China.COVID-19's diagnosis and treatment Plan (trial Seventh Edition). http://www.nhc.gov.cn/yzygj/s7653p/202002/d4b895337e19445f8d728fcaf1e3e13a.shtml.
- Peiris J.S.M., Chu C.M., Cheng V.C.C., Chan K.S., Hung I.F.N., Poon L.L.M. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryon J.J., Moss W.J., Monze M., Griffin D.E. Functional and phenotypic changes in circulating lymphocytes from hospitalized Zambian children with measles. Clin Diagn Lab Immunol. 2002;9:994–1003. doi: 10.1128/CDLI.9.5.994-1003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R., Champiat S., Dercle L., Aspeslagh S., Castanon E., Limkin E.J. Base-line lymphopenia should not be used as exclusion criteria in early clinical trials investigating immune checkpoint blockers (PD-1/PD-L1 inhibitors) Eur J Cancer. 2017;84:202–211. doi: 10.1016/j.ejca.2017.07.033. [DOI] [PubMed] [Google Scholar]
- Wong T., Fonseca K., Chernesky M.A., Garceau R., Levett P.N., Serhir B. Canadian Public Health Laboratory Network laboratory guidelines for the diagnosis of neurosyphilis in Canada. Can J Infect Dis Med Microbiol. 2015;26:18–22. doi: 10.1155/2015/167484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. WHO Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020[EB/OL] https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 [12.02.20] [Google Scholar]
- Xu Z., Shi L., Wang Y.J., Huang L., Zhang C., Liu S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Dong X., Cao Y., Yuan Y., Yang Y., Yan Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020:1–12. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]