Figure 5: The altered pharmacology of ERmuts is only evident when expressed at a level higher than the WT receptor:
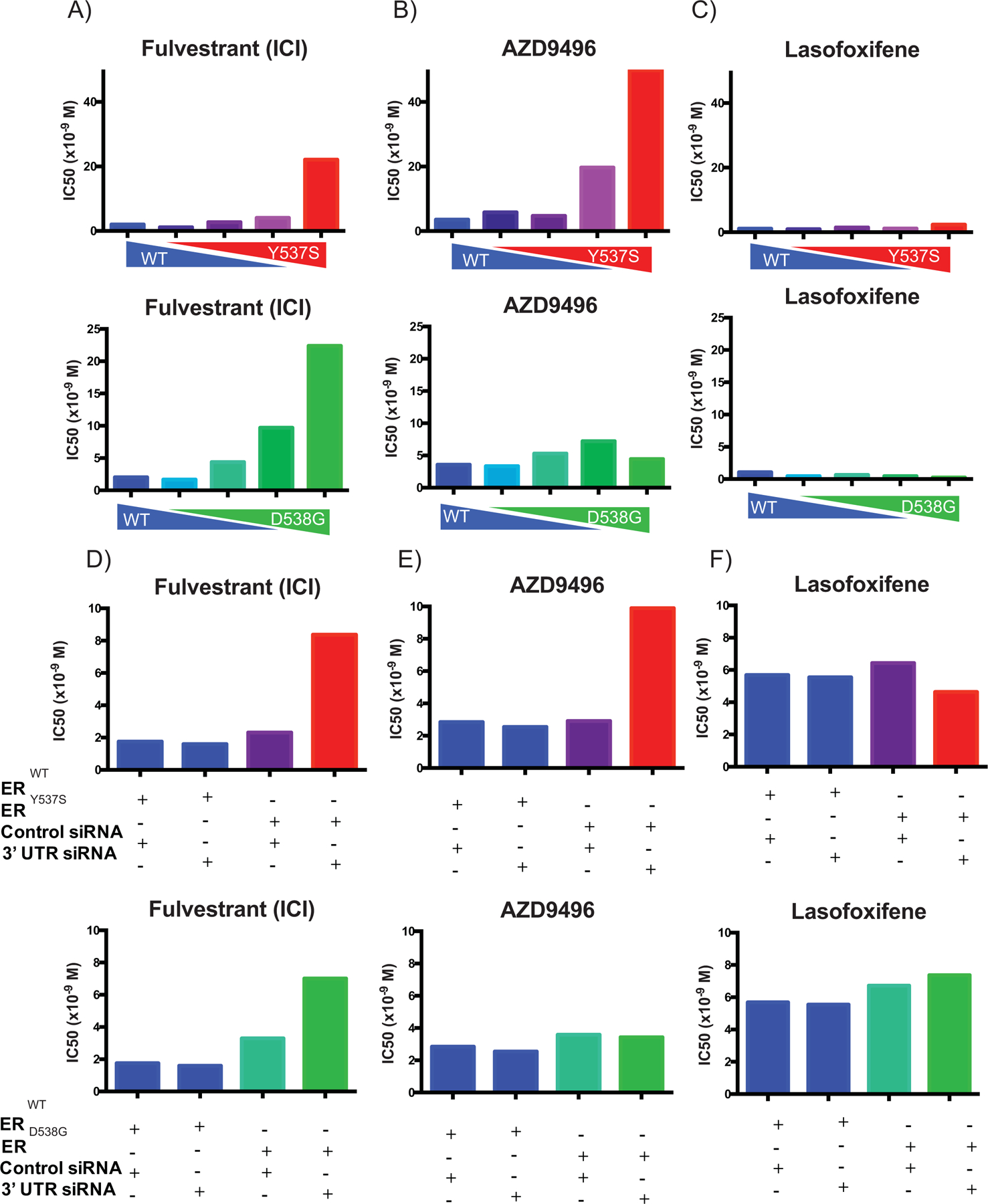
(A-C) SKBR3 (ER-negative breast cancer) cells plated in phenol red-free media supplemented with charcoal stripped serum and transfected with an estrogen responsive reporter gene (3X-ERE-tata-Luc) in the presence of different WT to mutant ER (Y537S or D538G) construct ratios. After 5 hours, cells were treated with 17β-estradiol (1 nM) and ER antagonists (10−12 M to 10−6 M). Firefly luciferase activity was assessed and normalized to β-galactosidase transfection control. The IC50s of each dose-response curve are plotted. Two-way ANOVA was utilized, comparing the logIC50s of all three independent experiments, to determine if there were significant differences between the WT and mutant receptors. Significant differences (p-value < 0.05) of the mutant IC50s when compared to that of the WT that were determined by this analysis are represented with a star. (D-F) MCF7 (ER-positive breast cancer) cells were engineered to express the WT or mutant receptors in response to doxycycline treatment. Cells were plated in phenol red-free media for 48 hours with doxycycline and siRNA (control or targeting 3’ UTR to knockdown the endogenous WT receptor) and then transfected with an estrogen responsive reporter gene (7X-ERE-tata-Luc). After 5 hours, cells were treated with 17β- estradiol (0.1 nM) and ER antagonists (10−12 M to 10−6 M). Firefly luciferase activity was assessed and normalized to β-galactosidase transfection control. The IC50s of each dose response curve are plotted. Two-way ANOVA was utilized, comparing the logIC50s of all three independent experiments, to determine if there were significant differences between the WT and mutant receptors. Significant differences (p-value < 0.05) of the mutant IC50s when compared to that of the WT that were determined by this analysis are represented with a star. Data presented is a representative of three independent experiments.
