Abstract
Glycemic variability (GV), defined as an integral component of glucose homoeostasis, is emerging as an important metric to consider when assessing glycemic control in clinical practice. Although it remains yet no consensus, accumulating evidence has suggested that GV, representing either short-term (with-day and between-day variability) or long-term GV, was associated with an increased risk of diabetic macrovascular and microvascular complications, hypoglycemia, mortality rates and other adverse clinical outcomes. In this review, we summarize the adverse clinical outcomes of GV and discuss the beneficial measures, including continuous glucose monitoring, drugs, dietary interventions and exercise training, to improve it, aiming at better addressing the challenging aspect of blood glucose management.
Keywords: Glycemic variability, Short-term glycemic variability, Long-term glycemic variability, Adverse clinical outcomes, Beneficial measures
Background
Glycemic variability (GV), referring to oscillations in blood glucose levels, is usually defined by the measurement of fluctuations of glucose or other related parameters of glucose homoeostasis over a given interval of time (i.e., within a day, between days or longer term). Although HbA1c was traditionally considered as the gold standard for assessing glycemic control [1], GV is a more meaningful measure of glycemic control than HbA1c in clinical practice, and is without doubt now being recognized [2].
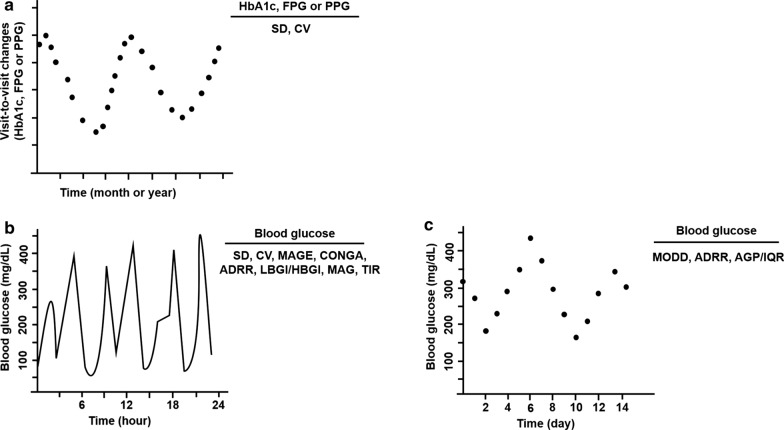
Despite its clinical significance, there is no consensus on the optimum method for characterizing GV [3]. Over the years, various metrics quantifying GV have been introduced, but many of them are not well understood [4, 5]. Thus, metrics effectively describing GV will be desirable. There are predominantly two types of GV according to the length of time-interval: long-term GV, based on serial determinations over a longer period of time, involving HbA1c, serial fasting plasma glucose (FPG) and postprandial glucose (PPG) measurements, and short-term GV, represented by both within-day and between-day GV (Table 1). Long-term GV, usually based on visit-to-visit measurements of HbA1c, FPG or PPG [6], with the subsequent calculation of their standard deviation (SD) and coefficient of variation (CV), reflects the surrounding hyperglycemia to a certain extent, because measures of long-term variability correlate with either mean concentration of blood glucose or mean HbA1c [7, 8] (Fig. 1a). For another type of GV, short-term GV is characterized by sudden and rapid upward or downward glucose changes within- or between-days (Fig. 1b, c). Furthermore, short-term GV is calculated from self-monitoring of blood glucose (SMBG) measurements for a long time [7], but this method has been gradually replaced by continuous glucose monitoring (CGM) over the past few years [9–12]. CGM, with interstitial glucose measurements at 5 min intervals, provides a more comprehensive record during the day and night periods compared to SMBG [7, 10]. Similar to long-term GV, the common metrics of short-term GV include the SD and CV. When averaging each daily SD or CV, the mean of within-day daily GV over the stated time period can also be estimated [4]. Service et al. introduced that the mean amplitude of glycemic excursions (MAGE) was the “gold standard” for assessing the short-term with-day GV [13]. Due to its simplicity, MAGE remained still commonly used to assess the with-day GV by further measuring the arithmetic mean of the differences between consecutive peaks and nadirs. Moreover, a novel approach to measurement of with-day GV was presented by the continuous overlapping net glycemic action (CONGA) metric that calculates the SD of difference between a current blood glucose reading and a reading taken hours earlier [14]. Another metric of with-day GV was the mean absolute glucose (MAG), which summed absolute differences between sequential readings divided by the time between the first and last blood glucose measurement [2]. Correspondingly, the metrics for estimating the between-day GV were referenced to as the mean of daily differences (MODD) and introduced by Molnar et al. [15]. This index assessed the between-day GV based on the calculation of the absolute differences between two glucose values measured at the same time with a 24 h interval. Average glucose profile (AGP), a measure of the between-day GV over a 14-day period, was determined by using the flash glucose monitoring system and reported the results as interquartile ranges (IQRs) [16, 17]. Apart from the above indices, particular attention should be given to the low blood glucose index (LBGI), high blood glucose index (HBGI) and average daily risk range (ADRR), as they were associated with the risk of hypo- and hyperglycemia. Among these indices, LBGI and HBGI were preceded by a log transform to render symmetric the skewed distribution of glucose values [4, 18], and ADRR was sum of the daily peak risks for hypo- and hyperglycemia [19]. Recently, time in range (TIR) was identified as a key metric of glycemic control, and referred to the percentage of time per day within target glucose range (3.9–10.0 mmol/L) [20, 21].
Table 1.
Main types of metric for assessment of GV
| Types of metric | Computation or description | References |
|---|---|---|
| Long-term GV | ||
| Visit-to-visit measurements of HbA1c, FPG or PPG | Measures of SD or CV of HbA1c, FPG and PPG between sequential visits | [6] |
| Short-term GV | ||
| SD | Variation around the mean blood glucose | [4] |
| CV | Magnitude of variability relative to mean blood glucose | [4] |
| MAGE | Mean differences from peaks to nadirs | [13] |
| CONGA | Difference between a current blood glucose reading and a reading taken hours earlier | [14] |
| MAG | Absolute differences between sequential readings divided by the time between the first and last blood glucose measurement | [2] |
| MODD | Absolute differences between two glucose values measured at the same time with a 24 h interval | [15] |
| AGP/IQR | Distribution of glucose data at a given timepoint and resulted as interquartile ranges | [16, 17] |
| LBGI/HBGI | Preceded by a log transform to render symmetric the skewed distribution of glucose values | [4, 18] |
| ADRR | Sum of the daily peak risks for hypoglycemia and hyperglycemia | [19] |
| TIR | Percentage of time per day within target glucose range (3.9–10.0 mmol/L) | [20, 21] |
GV, glycemic variability; FPG, fasting plasma glucose; PPG, postprandial glucose; SD, standard deviation; CV, coefficient of variation; MAGE, mean amplitude of glycemic excursions; CONGA, continuous overlapping net glycemic action; MAG, mean absolute glucose; MODD, mean of daily differences; AGP, average glucose profile; IQR, interquartile ranges; LBGI, low blood glucose index; HBGI, high blood glucose index; ADRR, average daily risk range; TIR, time in range
Fig. 1.
Two principal types of GV. a Long-term GV based on visit-to-visit changes of HbA1c, FPG or PPG. b, c Short-term GV represented by within-day and between-day GV. GV, glycemic variability; FPG, fasting plasma glucose; PPG, postprandial glucose; SD, standard deviation; CV, coefficient of variation; MAGE, mean amplitude of glycemic excursions; CONGA, continuous overlapping net glycemic action; MAG, mean absolute glucose; MODD, mean of daily differences; AGP, average glucose profile; IQR, interquartile ranges; LBGI, low blood glucose index; HBGI, high blood glucose index; ADRR, average daily risk range; TIR, time in range
In our previous study, we indicated that GV was correlated with cardiovascular events and hypoglycemia [22]. Although it remains yet controversial, emerging evidence has suggested that GV was associated with an increased risk of microvascular and macrovascular complications, hypoglycemia and mortality rates [23–25]. The aim of this study is to summarize the adverse clinical outcomes of GV and discuss the potential beneficial measures including CGM, drugs, diets and exercise to improve it, aiming to address the challenging aspect of blood glucose management.
GV and adverse clinical outcomes
Given that the limitations of HbA1c measurements, growing evidence demonstrated that GV was a significant and clinically meaningful glycemic metric and had drawn attention for its effects on adverse clinical outcomes, including diabetic macrovascular and microvascular complications, hypoglycemia and mortality [26–29] (Table 2).
Table 2.
The effects of GV on adverse clinical outcomes
| Types of GV | Subjects | Effects | References |
|---|---|---|---|
| Short-term GV | |||
| TIR | 3262 patients with type 2 diabetes | Inversely correlated with DR | [27] |
| Day-to-day FPG variability | 7637 patients with type 2 diabetes | Increased risks of severe hypoglycemia and all-cause mortality | [29] |
| MAGE | 417 patients with ACS | Predicted the poor prognosis for patients with acute coronary syndrome | [32] |
| Mean daily δ blood glucose | 160 patients with transcatheter aortic valve implantation | Increased the risk of macrovascular complications | [35] |
| MAGE | 204 patients with type 2 diabetes | Increased coronary artery disease severity | [36] |
| MAGE | 50 patients with dysglycemia | Positively correlated with coronary artery spasm | [37] |
| MAGE | 2666 hospitalized patients with CAD | Positively associated with poor prognosis in CAD patients | [38] |
| Incremental glucose peak | 2758 patients with type 2 diabetes | Positively associated with aortic stiffness and maladaptive carotid remodeling | [39] |
| MAGE | 40 patients with type 1 or type 2 diabetes | Positively associated with DPN | [51] |
| LBGI and HBGI | 140 patients with type 2 diabetes | Increased the risk of hypoglycemia | [66] |
| LBGI | 73 patients with type 1 diabetes | Increased the risk of hypoglycemia | [67] |
| Day‐to‐day fasting SMBG variability | 1221 patients with type 1 or type 2 diabetes | Increased the risk of overall symptomatic, nocturnal symptomatic and severe hypoglycemia | [68] |
| CONGA, MAG and MAGE | 83 patients with type 2 diabetes | Predicted the nocturnal hypoglycemia | [69] |
| Mean blood glucose | 62 patients with type 2 diabetes | Predicted the hypoglycemia | [70] |
| CV within a day | 6101 critically ill adults | Increased the risk of mortality and hypoglycemia | [72] |
| IQR | 28,353 patients with type 2 diabetes | Increased the risk of mortality | [73] |
| Long-term GV | |||
| Visit-to-visit variability of FPG | 654 patients with type 2 diabetes | Predicted the renal composite outcome | [31] |
| SD during initial hospitalization | 327 patients with diabetes and ACS | Predicted the midterm macrovascular complications | [40] |
| Visit-to-visit variability of FPG | 53,607 patients initially without CVD | Increased the risk of CVD and all-cause mortality | [41] |
| Visit-to-visit variability of FPG | 1791 patients with type 2 diabetes | Positively associated with the risk of CVD | [42] |
| Visit-to-visit variability of FPG | 455 patients with type 2 diabetes | Independently associated with annualized changes in left cardiac structure and function | [43] |
| Visit-to-visit variability of FPG | 3769 patients initially without CVD | Increased the risk of incident diabetes, CVD and mortality | [44] |
| Visit-to-visit variability of FPG | 3,211,319 patients without diabetes | Independently associated with CVD and mortality | [45] |
| Visit-to-visit variability of HbA1c | 632 patients with type 2 diabetes | Predicted the additive risk for CVD incidence | [46] |
| Visit-to-visit variability of HbA1c | 972 patients with type 2 diabetes | Positively associated with macrovascular complication | [47] |
| Visit-to-visit variability of HbA1c | 201 patients with type 2 diabetes | Potentially predicted the progression of HFpEF | [48] |
| Visit-to-visit variability of HbA1c | 902 patients with type 2 diabetes and heart failure | Predicted all-cause mortality | [49] |
| Visit-to-visit variability of FPG | 2773 patients with type 2 diabetes | Positively correlated with DPN | [52] |
| Visit-to-visit variability of FPG | 36,152 patients with type 2 diabetes | Predicted the risk of DPN | [53] |
| Visit-to-visit variability of HbA1c | 563 patients with type 2 diabetes | Positively associated the risk of DPN | [54] |
| Visit-to-visit variability of HbA1c | 220 patients with type 1 diabetes | Positively associated the risk of DPN | [55] |
| Visit-to-visit variability of HbA1c | 223 patients with type 2 diabetes | Positively associated with the severity of DPN | [56] |
| Visit-to-visit variability of HbA1c | 451 patients with type 1 diabetes | Increased the risk of DR | [58] |
| Visit-to-visit variability of HbA1c | 895 patients with type 2 diabetes | Positively associated with progression of DN | [60] |
| Visit-to-visit variability of HbA1c | 4231 patients with type 2 diabetes | Increased the risk of DKD | [61] |
| Visit-to-visit variability of HbA1c | 1383 patients with type 2 diabetes | Increased the deterioration of renal function | [62] |
| Visit-to-visit variability of HbA1c | 388 patients with type 2 diabetes | Positively associated with renal progression | [64] |
| Visit-to-visit variability of FPG | 3569 patients with type 2 diabetes | Increased the risk of mortality | [74] |
| Visit-to-visit variability of HbA1c | 15,733 patients with type 2 diabetes | Strongly predicted all-cause mortality | [75] |
| Visit-to-visit variability of FPG | 1136 patients with type 2 diabetes | Predicted all-cause mortality | [76] |
| Visit-to-visit variability of FPG | 42,418 hypertensive patients | Increased the risk of mortality | [77] |
| CV and SD during hospitalization | 20,303 hospitalized patients | Increased longer hospitalization and mortality | [78] |
| Visit-to-visit variability of HbA1c | 6048 patients with type 1 diabetes | Increased mortality and earlier hospital admission | [79] |
| Visit-to-visit variability of HbA1c | 58,832 patients with type 2 diabetes | Positively associated with overall mortality and emergency hospitalization | [80] |
| Visit-to-visit variability of HbA1c | 9483 patients with type 2 diabetes | Predicted all-cause mortality | [81] |
| Visit-to-visit variability of HbA1c | 837 patients with type 2 diabetes | Predicted depressive symptoms | [83] |
| Visit-to-visit variability of FPG | 3307 adults before the onset of diabetes | Increased the risk of cognitive function | [84] |
| Visit-to-visit variability of HbA1c | 2640 patients with type 1 or type 2 diabetes | Increased the potential risk of later tumorigenesis | [86] |
GV, glycemic variability; TIR, time in range; DR, diabetic retinopathy; FPG, fasting plasma glucose; MAGE, mean amplitude of glycemic excursions; ACS, acute coronary syndrome; CAD, coronary artery disease; LBGI, low blood glucose index; HBGI, high blood glucose index; SMBG, self‐monitored blood glucose; CONGA, continuous overlapping net glycemic action; MAG, mean absolute glucose; CV, coefficient of variation; IQR, interquartile ranges; CVD, cardiovascular disease; HFpEF, heart failure with preserved ejection fraction; DPN, diabetes peripheral neuropathy; DR, diabetic retinopathy; DN, diabetic nephropathy; DKD, diabetic kidney disease; SD, standard deviation
GV and diabetic macrovascular and microvascular complications
There is considerable evidence to support the negative role of GV in the development of diabetic macrovascular and microvascular complications [22, 30–33].
GV and diabetic macrovascular complications
An observational trial indicated that GV assessed by the MAGE was an independent predictive factor of poor prognosis for patients with acute coronary syndrome [32]. Moreover, a meta-analysis conducted by Liang et al. reported that high amplitude of GV played a causal role in cardiovascular disease (CVD), and minimizing GV could improve insulin resistance and reduce carotid intima-media thickness, as well as lower the risk of CVD [34]. Similarly, a post hoc cohort analysis showed that GV evaluated by mean daily δ blood glucose was associated with an increased risk of macrovascular complications (e.g., death, stroke and myocardial infarction) after transcatheter aortic valve implantation [35]. In acute myocardial infraction patients with poorly controlled type 2 diabetes, GV represented by MAGE was associated with coronary artery disease (CAD) severity, and suggested that early evaluation of GV might serve as a therapeutic target [36]. Particularly, intraday GV was thought to be associated with coronary artery spasm in patients with dysglycemia [37]. Recently, Pu et al. showed that increased GV on admission might be associated with poor prognosis in CAD patients [38]. Of note, a current study indicated that daily glucose variability represented by incremental glucose peak during an oral glucose tolerance test was independently associated with aortic stiffness and maladaptive carotid remodeling, but not with microvascular function [39].
In addition to the short-term GV, long-term GV was also strongly associated with the macrovascular complications. Gerbaud et al. reported that GV (cutoff value > 2.70 mmol/L) assessed by SD during initial hospitalization was the strongest independent predictive factor for midterm macrovascular complications in patients with diabetes and acute coronary syndrome [40]. A prospective cohort study including 53,607 Chinese participants found that long-term visit-to-visit variability of FPG increased the risk of CVD (included myocardial infarction, cerebral infarction, and cerebral hemorrhage) and all-cause mortality [41]. In the Veteran Affairs Diabetes Trial (VADT), the adverse consequences of FPG variability on CVD, mainly including myocardial infarction, stroke and cardiovascular death, appeared greatest in patients receiving intensive glucose control [42]. Even more important, visit-to-visit variability in FPG could be a novel risk factor for the long-term adverse changes in left cardiac structure and systolic function [43]. Currently, Bancks et al. suggested that higher intra-individual FPG variability during young adulthood before the onset of diabetes was associated with incident diabetes, macrovascular events and mortality [44]. Noteworthily, Yu et al. even found that long-term FPG variability was independently associated with myocardial infarction and stroke in a general population without diabetes [45]. In addition to the variability of FPG, long-term variability of HbA1c was also correlated with the risk of macrovascular complications. A previous study investigated the association of long-term variability of HbA1c and systolic blood pressure with the incidence of macrovascular complications in patients with type 2 diabetes, and found that they represented a combined and additive risk for macrovascular complications [46]. Moreover, a study identified that long-term variability of HbA1c was associated with macrovascular complication in Chinese type 2 diabetes [47]. Meaningfully, HbA1c variability may provide additional valuable information as a potential predictor for the progression of heart failure with preserved ejection fraction (HFpEF) [48], and was independently and similarly predictive of death or HFpEF [49]. Moreover, GV evaluated by SD of blood glucose level appeared to be an important risk factor for left ventricular diastolic function, and reducing GV may provide a potential new therapeutic strategy for the prevention of the development of HFpEF in T2DM patients [50].
GV and diabetic microvascular complications
Likewise, GV played an important role in diabetic microvascular complications. In the Rio De Janeiro Type 2 Diabetes Cohort Study, 24-month visit-to-visit FPG variability was a significant risk predictor for renal outcomes, and 24-month visit-to-visit HbA1c variability was a better risk predictor for diabetic retinopathy progression than HbA1c levels [31]. Akaza et al. revealed that GV estimated by MAGE might be an independently risk factor for diabetes peripheral neuropathy (DPN) in patients with type 1 or type 2 diabetes by using CGM [51]. Specially, long-term FPG variability as represented by the CV was related to the risk of DPN in patients with type 2 diabetes [52]. More importantly, in the National Diabetes Care Management Program, the long-term variability of FPG was considered as one of the potent predictors of DPN in type 2 diabetic patients [53]. On the other hand, researchers disclosed that long-term variability of HbA1c assessed by CV was closely associated with DPN, and was identified as an indicator for DPN in type 1 or type 2 diabetes [54, 55]. Lai et al. performed a cross-sectional study enrolled 223 patients with type 2 diabetes and demonstrated that 3-year visit-to-visit HbA1c variability combined with chronic glycemic impairment was strongly associated with the severity of DPN [56]. They also confirmed that HbA1c variability was independently associated with the severity of cardiovascular autonomic neuropathy [57]. Intriguingly, a recent study showed that GV involved in long-term visit-to-visit HbA1c variability was independently associated with the risk of diabetic retinopathy (DR) in type 1 diabetes [58]. Consistently, Lu et al. revealed that GV estimated by TIR was also strongly associated with DR in patients with type 2 diabetes [27]. Furthermore, a systematic review and meta-analysis ascertained that high FPG variability levels were positively associated with the risk of DR and all-cause mortality in patients with type 2 diabetes [59]. Apart from the DR, GV represented by long-term variability of HbA1c was also significantly associated with the progression of diabetic nephropathy (DN) in type 2 diabetes [60]. The long-term variability of HbA1c, lipid parameters, uric acid and blood pressure influenced the development of DN and had a different impact on albuminuria development and the decline in glomerular filtration rate [61, 62]. Subsequent research clarified that the long-term intra-individual variability in these parameters played a greater role in the progression of DN than the absolute value of each single variable [63]. Importantly, Lee et al. demonstrated that greater HbA1c variability and a decreasing trend of HbA1c was associated with a lower risk of diabetic patients with stages 3–4 chronic kidney disease and poor glycemic control [64]. These findings collectively displayed the pivotal role of GV in diabetic macrovascular and microvascular complications.
GV and hypoglycemia
Hypoglycemia is the major impediment to therapy in diabetes. While HbA1c remains widely used as a measure of mean glycemia, it may not be the best marker for predicting hypoglycemia. The consolidated evidence to date supported the importance of GV with respect to predicted risk of hypoglycemia [65–67]. Zinman et al. concluded that higher day-to-day FPG variability was associated with increased risks of severe hypoglycemia and all-cause mortality [29]. Moreover, day-to-day fasting SMBG variability was also found to be associated with the risk of overall symptomatic, nocturnal symptomatic and severe hypoglycemia in insulin-treated patients with diabetes [68]. Similarly, the analysis of CGM-derived GV could improve prediction of nocturnal hypoglycemia in elderly patients treated with insulin, and minimizing GV could achieve good glycemic control without hypoglycemia [69, 70]. Additionally, using nested case–control design in electronic health record data in England, Zhong et al. showed that HbA1C variability is a strong predictor for hypoglycemia requiring hospitalization in diabetes [71]. Overall, GV variability may be an important target for hypoglycemia prevention and management in diabetic patients treated with insulin.
GV and mortality
A number of studies verified that GV was not only associated with the risk of diabetes-related complications and hypoglycemia, but also simultaneously related to the high incidence of mortality [41, 44, 57]. Interestingly, several studies proposed an independent association of GV with mortality [72–75]. Clinical data indicated that FPG variability might be an important predictor of mortality, particularly for those with their glycemic status uncontrolled [76, 77]. Besides, in hospitalized patients, increased GV was associated with a higher rate of mortality [78–80]. Recently, in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, researchers found that HbA1c variability was a strong predictor of all-cause mortality [81], and this observation was more remarkable in older people with diabetes [82].
In addition to the above adverse clinical outcomes, GV was also reported to be associated with depressive symptoms, cognitive disorder and even cancer [83–86]. In the Israel Diabetes and Cognitive Decline (IDCD) study, GV measured as the SD of HbA1c increased the risk of depressive symptoms [83]. A Taiwan diabetes study explored the relationship between GV and the incidence of Alzheimer disease (AD) in patients with type 2 diabetes mellitus, finding that GV had a worse impact on AD and might be significant predictors for AD [84]. More importantly, recent study demonstrated that HbA1c variability was a potential risk factor for later tumorigenesis in patients with diabetes, which might be mediated by oxidative stress or hormone variability [86].
Potential beneficial measures
There is now cogent evidence for the deleterious effects of GV. As a consequence, it is strongly suggested that potential beneficial measures should be aimed at reducing to a minimum GV (Table 3).
Table 3.
Potential beneficial measures for addressing GV
| Subjects | Measures | Results | References |
|---|---|---|---|
| Patients with type 1 diabetes | CGM | Reduced GV and improved protection against hypoglycemia | [87–89] |
| Insulin analogues degludec | Minimized morning GV | [91] | |
| Canagliflozin | Improved indices of GV | [92] | |
| Dapagliflozin over 24 weeks | Improved GV without increasing the time spent in the range indicating hypoglycemia | [93] | |
| Empagliflozin as adjunct to insulin | Decreased glucose exposure and variability and increased time in glucose target range | [103] | |
| Combination of basal insulin with ipragliflozin or dapagliflozin | Improved TIR and the mean glucose level | [104] | |
| Low carbohydrate diet | Resulted in more time in euglycemia, less time in hypoglycemia | [108–110] | |
| Patients with type 2 diabetes | Dapagliflozin on 24-h | Improved measures of GV | [94] |
| Once-weekly trelagliptin and once-daily alogliptin | Improved glycemic control and reduced GV without inducing hypoglycemia | [95] | |
| Combination of basal insulin with a GLP-1 RA | Lowered GV and hypoglycemia | [96] | |
| Exenatide once weekly | Improved daily glucose control and reduced GV | [97] | |
| Lixisenatide added to basal insulin | Reduced GV and PPG excursions without increasing the risk of hypoglycemia | [98] | |
| Liraglutide | Lower mean time in hyperglycemia | [99] | |
| Combination of metformin and gemigliptin or sitagliptin | Significantly reduced GV | [100] | |
| Vildagliptin or pioglitazone | Significantly reduced MAGE, glycated hemoglobin and mean plasma glucose levels | [101] | |
| Combination of metformin and vildagliptin or glimepiride | Improved glucose level with a significantly greater reduction in GV and hypoglycemia | [102] | |
| Intensive insulin therapy combined with metformin | Reduced both glucose fluctuation and nocturnal hypoglycemic risk | [105] | |
| Low-carbohydrate high-fat diet | Reduced glycemic fluctuation | [106, 107, 111] | |
| Sequence of food ingestion | Associated with lower post-lunch glucose excursions and lower glucose coefficients of variation | [115] | |
| Aerobic and combined exercise sessions | Reduced glucose levels and GV | [116–118] | |
| Short-term exercise training | Improved glycemic control and GV but unaffected oxidative stress | [119, 121] | |
| Frequent interruptions of prolonged sitting | Improved fasting glucose and night-time glycemic variability | [120] | |
| Others | Low glycemic index foods | Reduced the glycemic response and variability and promoted fat oxidation. | [112, 113] |
| Food order | Reduced glycemic excursions | [114] | |
| Exercise in the fasted and postprandial state | Exercise in the postprandial state after breakfast, but not in the fasted state, decreased glucose excursions | [122] | |
| Aerobic and eccentric exercise | Reduced all the indices of GV | [123] | |
| Immediate post-breakfast physical activity | Improved mean, CV and AUC glucose | [124] |
GV, glycemic variability; CGM, continuous glucose monitoring; CV, coefficient of variation; GLP-1 RA, glucagon-like peptide 1 receptor agonist; PPG, postprandial glucose; MAGE, mean amplitude of glycemic excursions; TIR, time in range; AUC, area under the curve
Drugs combined with CGM
Extensive evidence addresses that real-time CGM (rtCGM) improves glycemic control and minimizes the risk of glucose extremes, as well as severe hypoglycemia [87–90]. rtCGM combined with drugs allows a comprehensive analysis of GV and makes timely adjustments. Treatment with insulin analogues degludec, in the context of GV measured by CGM, was related to the lower day-to-day variation in glucose level [91]. In randomized, double-blind studies, canagliflozin and dapagliflozin improved GV in the participants who underwent CGM [92–94]. Furthermore, another randomized pilot study indicated that once-weekly trelagliptin and once-daily alogliptin improved glycemic control and reduced GV without inducing hypoglycemia [95]. Nowadays, greater efficacy is shown in therapies combining new hypoglycemic drugs with insulin or metformin, with improvement in GV also demonstrated by CGM. Bajaj et al. revealed that the combination of glucagon-like peptide 1 receptor agonist (GLP-1 RA) with basal insulin observed the lowest GV and hypoglycemia in type 2 diabetes [96]. In metformin-treated patients with type 2 diabetes, exenatide once weekly significantly improved daily glucose control and reduced GV [97]. Analogously, compared with placebo, lixisenatide added to basal insulin significantly reduced GV and PPG excursions without increasing the risk of hypoglycemia [98]. Furthermore, for type 2 diabetes patients initially treated with insulin, introducing liraglutide had a beneficial effect on GV estimated by CGM [99]. Another new hypoglycemic drugs, dipeptidyl-peptidase 4 (DPP4) inhibitors, combined with metformin therapy improved glucose level with a significantly greater reduction in GV and hypoglycemia [100–102]. A multicenter study compared the GV between DPP4 inhibitor and glimepiride groups, and found that DPP4 inhibitors were more effective than glimepiride in reducing GV as initial combination therapy with metformin in patients with type 2 diabetes [100]. Moreover, other studies demonstrated that vildagliptin reduced GV in individuals with type 2 diabetes ongoing metformin therapy [101, 102]. Consistent results were obtained when combined sodium glucose cotransporter 2 (SGLT2) inhibitors with insulin therapy [103, 104]. Famulla et al. addressed that empagliflozin as adjunct to insulin decreased glucose exposure and variability and increased time in glucose target range in patients with type 1 diabetes [103]. A recent retrospective study unraveled that SGLT2 inhibitors improved TIR without increasing hypoglycemia in Japanese patients with type 1 diabetes [104]. Notably, an observational study indicated that metformin added to initial continuous subcutaneous insulin infusion or multiple daily injections decreased glucose fluctuation and nocturnal hypoglycemic risk in patients with type 2 diabetes [105]. These results clarified that new antidiabetic drugs combined with CGM might be the preferred choice for the reduction of GV.
Dietary interventions
As an important component of diabetes management, the impact of dietary manipulation on glycemic control cannot be understated. A previous study by Mori et al. demonstrated that the low-carbohydrate/high-monounsaturated fatty acid liquid diet narrowed the range of GV, and might be useful in long-term glycemic control [106]. The current study also compared the effect of glycemic response to low-carbohydrate high-fat diet and high-carbohydrate low-fat diet by using CGM, finding the consistent results [107]. Furthermore, low carbohydrate diet contributed to more time in euglycemia, less GV than high carbohydrate diet [108–110]. Particularly, a very-low-carbohydrate high-fat breakfast appeared to be sufficient to reduce postprandial hyperglycemia and improve glucose excursions [111]. Low glycemic index foods can minimize blood glucose fluctuations and have been advocated to use in diabetic patients. Henry et al. indicated that lower glycemic index foods were able to acutely reduce the GV and promote fat oxidation [112, 113]. Of note, a recent study suggested that the food order (protein or vegetables first, followed by carbohydrate) decreased GV in prediabetes, which presented a novel, simple behavioral strategy to reduce glycemic excursions [114, 115]. In short, effective dietary interventions have the potential to achieve a favorable blood glucose profile by influencing the GV.
Exercise training
Exercise training, consisting of resistance exercise, aerobic exercise, or a combination of both, is recognized as a frontline therapy for the prevention and treatment of type 2 diabetes. Additionally, previous studies showed that exercise reduced GV or oxidative stress [116, 117], opening a new venue of benefits to explore. There is evidence that different types of exercise have various effects on glucose control. Schein et al. performed a randomized clinical trial and found that inspiratory muscle training decreased glucose levels and GV in patients with type 2 diabetes, which could be a novel exercise modality [118]. Another crossover trial showed that short-term interval walking training improved CGM-derived GV compared with continuous walking training in individuals with type 2 diabetes [119]. Lately, Paining et al. explored that frequent interruptions of prolonged sitting with 3 min of light-intensity walking breaks every 15 min improved night-time GV, which might be an effective approach to improve glucose control [120]. Furthermore, 2 weeks of both high-intensity interval training or moderate-intensity continuous training were similarly effective in lowering GV and endothelial damage [121]. Intriguingly, a randomized study was to test whether moderate exercise performed in either the fasted or the postprandial state affected GV, and concluded that performing moderate exercise in the postprandial state after breakfast tended to decrease glucose excursions compared to the exercise in the fasted state [122].
Recently, in addition to the effect of exercise on GV in patients with diabetes, the same phenomenon was also observed in healthy people. Figueira et al. observed that both aerobic and eccentric exercise reduced GV in healthy individuals, which might be mediated by inflammatory cytokines [123]. Moreover, consistent with the results in patients with diabetes, low- to moderate-intensity exercise soon after breakfast improved GV in healthy people, which will help optimize exercise-meal timing in general health guidelines [124].
Conclusion and future perspective
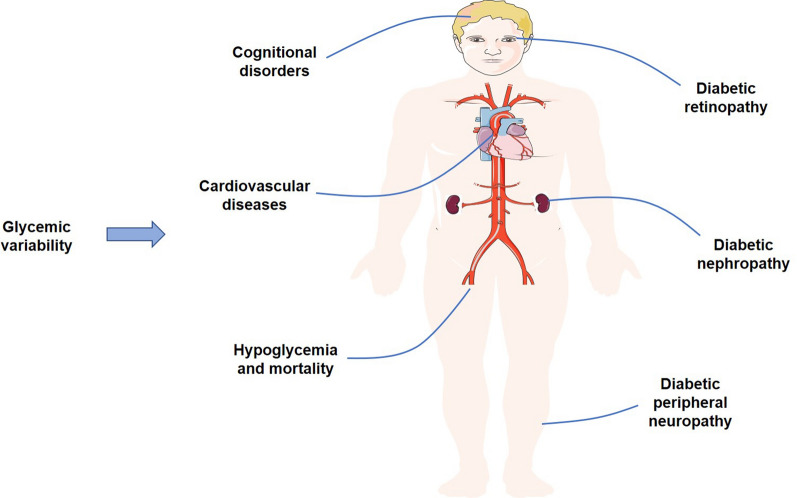
We have attempted to summarize the relationships between two categories of GV and the risk for diabetic macrovascular and microvascular complications, hypoglycemia, mortality and other adverse clinical outcomes (Fig. 2). We also generalized the potential beneficial measures including drugs combined with CGM, dietary interventions and exercise training, to improve GV. These findings highlight the important role of GV in the patients with diabetes and provide the essential help for clinicians to manage the blood glucose.
Fig. 2.
The effects of glycemic variability on the adverse clinical outcomes
GV has been identified to be closely associated with the risk of adverse clinical outcomes and provides a better predictor of such complications. However, it still lacking a clear universal definition and different indices have been proposed to evaluate it. With the availability of CGM in clinical practice, the assessment of GV became not only possible but also required [2]. Also, CGM was frequently superior to continuous subcutaneous insulin infusion and could guide individuals’ therapeutic changes to reduce GV, hypoglycemia and CVD [125, 126]. A recent study reported that flash glucose monitoring, a new approach to glucose monitoring, has a long sensor lifetime of 14 days and emerged as a practical solution to the glucose monitoring [127]. Meanwhile, a real-world data from Spain indicated that flash glucose monitoring allowed frequent glucose checks and reduced GV, as well as hypoglycemia [128]. Consequently, in order to provide a more comprehensive assessment of GV, the new approach of glucose monitoring is advocated to adopt in clinical practice. Future developments in new technologies, such as CGM systems and flash glucose monitoring, and indices for better deciphering and defining GV should contribute to improve understanding of the clinical relevance of GV in the management of diabetes.
Although GV had drawn attention for its effects on diabetic macrovascular and microvascular complications, hypoglycemia and mortality, several studies have shown conflicting results [7, 129]. Caprnda et al. failed to show the association between diabetic complication and GV in patients with type 2 diabetes [129]. Furthermore, in the Diabetes Control and Complications Trial, within-day GV, as determined from quarterly glucose profiles, did not play an explicit role in the development of microvascular complications [7]. However, we found that these results employed the 7-point glucose profiles, which might be insufficient to characterize GV correctly when compared with CGM. Thus, these negative results may not necessarily disprove the importance of GV in the development of diabetic complications. Additionally, the mechanisms linking GV and related complications risk remained unclear. Recent studies corroborated that GV was correlated with oxidative stress or erythrocyte membrane stability, emphasizing its participation in the pathogenesis of related complications [130, 131]. Further prospective research to explore the explicit mechanisms linking GV and related complications is warranted.
Finally, setting clear definitions and taking potential beneficial measures for addressing GV is essential. Further research in these domains will contribute to blood glucose control and management.
Acknowledgements
The authors wish to acknowledge Xiaochuan Zhang from the First Affiliated Hospital of Zhengzhou University, China for editing of English grammar and syntax of the manuscript.
Abbreviations
- GV
glycemic variability
- FPG
fasting plasma glucose
- PPG
postprandial glucose
- SD
standard deviation
- CV
coefficient of variation
- SMBG
self-monitoring of blood glucose
- CGM
continuous glucose monitoring
- MAGE
mean amplitude of glycemic excursions
- CONGA
continuous overlapping net glycemic action
- MAG
mean absolute glucose
- MODD
mean of daily differences
- AGP
average glucose profile
- IQRs
interquartile ranges
- LBGI
low blood glucose index
- HBGI
high blood glucose index
- ADRR
average daily risk range
- TIR
time in range
- CVD
cardiovascular disease
- CAD
coronary artery disease
- VADT
Veteran Affairs Diabetes Trial
- HFpEF
heart failure with preserved ejection fraction
- DPN
diabetes peripheral neuropathy
- DR
diabetic retinopathy
- DN
diabetic nephropathy
- ACCORD
Action to Control Cardiovascular Risk in Diabetes
- IDCD
Israel Diabetes and Cognitive Decline
- AD
Alzheimer disease
- rtCGM
real-time continuous glucose monitoring
- GLP-1 RA
glucagon-like peptide 1 receptor agonist
- DPP4
dipeptidyl-peptidase 4
- SGLT2
sodium glucose cotransporter 2
Authors’ contributions
All authors participated in the writing and editing of this review article. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have approved the final manuscript for publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zheng Zhou and Bao Sun contributed equally to this work
Contributor Information
Chunsheng Zhu, Email: zhuchunsheng6@163.com.
Meng Bian, Email: bianmeng0208@163.com.
References
- 1.Nathan DM, Turgeon H, Regan S. Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia. 2007;50(11):2239–2244. doi: 10.1007/s00125-007-0803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeVries JH. Glucose variability: where it is important and how to measure it. Diabetes. 2013;62(5):1405–1408. doi: 10.2337/db12-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frontoni S, Di Bartolo P, Avogaro A, Bosi E, Paolisso G, Ceriello A. Glucose variability: an emerging target for the treatment of diabetes mellitus. Diabetes Res Clin Pract. 2013;102(2):86–95. doi: 10.1016/j.diabres.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Kovatchev BP. Metrics for glycaemic control—from HbA1c to continuous glucose monitoring. Nat Rev Endocrinol. 2017;13(7):425–436. doi: 10.1038/nrendo.2017.3. [DOI] [PubMed] [Google Scholar]
- 5.Bergenstal RM. Glycemic variability and diabetes complications: does it matter? simply put, there are better glycemic markers! Diabetes Care. 2015;38(8):1615–1621. doi: 10.2337/dc15-0099. [DOI] [PubMed] [Google Scholar]
- 6.Gorst C, Kwok CS, Aslam S, Buchan I, Kontopantelis E, Myint PK, Heatlie G, Loke Y, Rutter MK, Mamas MA. Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care. 2015;38(12):2354–2369. doi: 10.2337/dc15-1188. [DOI] [PubMed] [Google Scholar]
- 7.Lachin JM, Bebu I, Bergenstal RM, Pop-Busui R, Service FJ, Zinman B, Nathan DM. Association of glycemic variability in type 1 diabetes with progression of microvascular outcomes in the diabetes control and complications trial. Diabetes care. 2017;40(6):777–783. doi: 10.2337/dc16-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kilpatrick ES, Rigby AS, Atkin SL. A1C variability and the risk of microvascular complications in type 1 diabetes: data from the Diabetes Control and Complications Trial. Diabetes Care. 2008;31(11):2198–2202. doi: 10.2337/dc08-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill NR, Oliver NS, Choudhary P, Levy JC, Hindmarsh P, Matthews DR. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol Ther. 2011;13(9):921–928. doi: 10.1089/dia.2010.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, Garg S, Heinemann L, Hirsch I, Amiel SA, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–1640. doi: 10.2337/dc17-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borot S, Benhamou PY, Atlan C, Bismuth E, Bonnemaison E, Catargi B, Charpentier G, Farret A, Filhol N, Franc S, et al. Practical implementation, education and interpretation guidelines for continuous glucose monitoring: a French position statement. Diabetes Metab. 2018;44(1):61–72. doi: 10.1016/j.diabet.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Petrie JR, Peters AL, Bergenstal RM, Holl RW, Fleming GA, Heinemann L. Improving the Clinical Value and Utility of CGM Systems: issues and Recommendations: A Joint Statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetes Care. 2017;40(12):1614–1621. doi: 10.2337/dci17-0043. [DOI] [PubMed] [Google Scholar]
- 13.Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19(9):644–655. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 14.McDonnell CM, Donath SM, Vidmar SI, Werther GA, Cameron FJ. A novel approach to continuous glucose analysis utilizing glycemic variation. Diabetes Technol Ther. 2005;7(2):253–263. doi: 10.1089/dia.2005.7.253. [DOI] [PubMed] [Google Scholar]
- 15.Molnar GD, Taylor WF, Ho MM. Day-to-day variation of continuously monitored glycaemia: a further measure of diabetic instability. Diabetologia. 1972;8(5):342–348. doi: 10.1007/BF01218495. [DOI] [PubMed] [Google Scholar]
- 16.Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther. 2015;17(11):787–794. doi: 10.1089/dia.2014.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoss U, Budiman ES. Factory-calibrated continuous glucose sensors: the science behind the technology. Diabetes Technol Ther. 2017;19:S44–S50. doi: 10.1089/dia.2017.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Clarke W. Symmetrization of the blood glucose measurement scale and its applications. Diabetes Care. 1997;20(11):1655–1658. doi: 10.2337/diacare.20.11.1655. [DOI] [PubMed] [Google Scholar]
- 19.Fabris C, Patek SD, Breton MD. Are risk indices derived from CGM interchangeable with SMBG-based indices? J Diabetes Sci Technol. 2015;10(1):50–59. doi: 10.1177/1932296815599177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, Bosi E, Buckingham BA, Cefalu WT, Close KL, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun B, He F, Gao Y, Zhou J, Sun L, Liu R, Xu H, Chen X, Zhou H, Liu Z, et al. Prognostic impact of visit-to-visit glycemic variability on the risks of major adverse cardiovascular outcomes and hypoglycemia in patients with different glycemic control and type 2 diabetes. Endocrine. 2019;64(3):536–543. doi: 10.1007/s12020-019-01893-1. [DOI] [PubMed] [Google Scholar]
- 23.Hirakawa Y, Arima H, Zoungas S, Ninomiya T, Cooper M, Hamet P, Mancia G, Poulter N, Harrap S, Woodward M, et al. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: the ADVANCE trial. Diabetes Care. 2014;37(8):2359–2365. doi: 10.2337/dc14-0199. [DOI] [PubMed] [Google Scholar]
- 24.Folli F, Corradi D, Fanti P, Davalli A, Paez A, Giaccari A, Perego C, Muscogiuri G. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: avenues for a mechanistic-based therapeutic approach. Curr Diabetes Rev. 2011;7(5):313–324. doi: 10.2174/157339911797415585. [DOI] [PubMed] [Google Scholar]
- 25.Bruginski D, Precoma DB, Sabbag A, Olandowski M. Impact of glycemic variability and hypoglycemia on the mortality and length of hospital stay among elderly patients in Brazil. Curr Diabetes Rev. 2020;16(2):171–180. doi: 10.2174/1573399815999190619141622. [DOI] [PubMed] [Google Scholar]
- 26.Slieker RC, van der Heijden A, Nijpels G, Elders PJM. Visit-to-visit variability of glycemia and vascular complications: the Hoorn Diabetes Care System cohort. Cardiovasc Diabetol. 2019;18(1):170. doi: 10.1186/s12933-019-0975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu J, Ma X, Zhou J, Zhang L, Mo Y, Ying L, Lu W, Zhu W, Bao Y, Vigersky RA, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care. 2018;41(11):2370–2376. doi: 10.2337/dc18-1131. [DOI] [PubMed] [Google Scholar]
- 28.Picconi F, Parravano M, Ylli D, Pasqualetti P, Coluzzi S, Giordani I, Malandrucco I, Lauro D, Scarinci F, Giorno P, et al. Retinal neurodegeneration in patients with type 1 diabetes mellitus: the role of glycemic variability. Acta Diabetol. 2017;54(5):489–497. doi: 10.1007/s00592-017-0971-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zinman B, Marso SP, Poulter NR, Emerson SS, Pieber TR, Pratley RE, Lange M, Brown-Frandsen K, Moses A, Ocampo Francisco AM, et al. Day-to-day fasting glycaemic variability in DEVOTE: associations with severe hypoglycaemia and cardiovascular outcomes (DEVOTE 2) Diabetologia. 2018;61(1):48–57. doi: 10.1007/s00125-017-4423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nusca A, Tuccinardi D, Albano M, Cavallaro C, Ricottini E, Manfrini S, Pozzilli P, Di Sciascio G. Glycemic variability in the development of cardiovascular complications in diabetes. Diab Metab Res Rev. 2018;34(8):e3047. doi: 10.1002/dmrr.3047. [DOI] [PubMed] [Google Scholar]
- 31.Cardoso CRL, Leite NC, Moram CBM, Salles GF. Long-term visit-to-visit glycemic variability as predictor of micro- and macrovascular complications in patients with type 2 diabetes: the Rio de Janeiro Type 2 Diabetes Cohort Study. Cardiovasc Diabetol. 2018;17(1):33. doi: 10.1186/s12933-018-0677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi H, Iwahashi N, Kirigaya J, Kataoka S, Minamimoto Y, Gohbara M, Abe T, Okada K, Matsuzawa Y, Konishi M, et al. Glycemic variability determined with a continuous glucose monitoring system can predict prognosis after acute coronary syndrome. Cardiovasc Diabetol. 2018;17(1):116. doi: 10.1186/s12933-018-0761-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia J, Yin C. Glucose variability and coronary artery disease. Heart Lung Circ. 2019;28(4):553–559. doi: 10.1016/j.hlc.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 34.Liang S, Yin H, Wei C, Xie L, He H, Liu X. Glucose variability for cardiovascular risk factors in type 2 diabetes: a meta-analysis. J Diab Metab Disord. 2017;16:45. doi: 10.1186/s40200-017-0323-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Besch G, Pili-Floury S, Morel C, Gilard M, Flicoteaux G, Mont L, Perrotti A, Meneveau N, Chocron S, Schiele F, et al. Impact of post-procedural glycemic variability on cardiovascular morbidity and mortality after transcatheter aortic valve implantation: a post hoc cohort analysis. Cardiovasc Diabetol. 2019;18(1):27. doi: 10.1186/s12933-019-0831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benalia M, Zeller M, Mouhat B, Guenancia C, Yameogo V, Greco C, Yao H, Maza M, Verges B, Cottin Y. Glycaemic variability is associated with severity of coronary artery disease in patients with poorly controlled type 2 diabetes and acute myocardial infarction. Diab Metab. 2019;45(5):446–452. doi: 10.1016/j.diabet.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Ito T, Ichihashi T, Fujita H, Sugiura T, Yamamoto J, Kitada S, Nakasuka K, Kawada Y, Ohte N. The impact of intraday glucose variability on coronary artery spasm in patients with dysglycemia. Heart Vessels. 2019;34(8):1250–1257. doi: 10.1007/s00380-019-01353-w. [DOI] [PubMed] [Google Scholar]
- 38.Pu Z, Lai L, Yang X, Wang Y, Dong P, Wang D, Xie Y, Han Z. Acute glycemic variability on admission predicts the prognosis in hospitalized patients with coronary artery disease: a meta-analysis. Endocrine. 2020;67(3):526–534. doi: 10.1007/s12020-019-02150-1. [DOI] [PubMed] [Google Scholar]
- 39.Foreman YD, Brouwers M, Berendschot T, van Dongen M, Eussen S, van Greevenbroek MMJ, Henry RMA, Houben A, van der Kallen CJH, Kroon AA, et al. The oral glucose tolerance test-derived incremental glucose peak is associated with greater arterial stiffness and maladaptive arterial remodeling: the Maastricht Study. Cardiovasc Diabetol. 2019;18(1):152. doi: 10.1186/s12933-019-0950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerbaud E, Darier R, Montaudon M, Beauvieux MC, Coffin-Boutreux C, Coste P, Douard H, Ouattara A, Catargi B. Glycemic . Diabetes Care. 2019;42(4):674–681. doi: 10.2337/dc18-2047. [DOI] [PubMed] [Google Scholar]
- 41.Wang A, Liu X, Xu J, Han X, Su Z, Chen S, Zhang N, Wu S, Wang Y, Wang Y. Visit-to-visit variability of fasting plasma glucose and the risk of cardiovascular disease and all-cause mortality in the general population. J Am Heart Assoc. 2017;6:12. doi: 10.1161/JAHA.117.006757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou JJ, Schwenke DC, Bahn G, Reaven P. Glycemic variation and cardiovascular risk in the veterans affairs diabetes trial. Diabetes Care. 2018;41(10):2187–2194. doi: 10.2337/dc18-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang X, Zhong J, Zhang H, Luo Y, Liu X, Peng L, Zhang Y, Qian X, Jiang B, Liu J, et al. Visit-to-visit fasting plasma glucose variability is an important risk factor for long-term changes in left cardiac structure and function in patients with type 2 diabetes. Cardiovasc Diabetol. 2019;18(1):50. doi: 10.1186/s12933-019-0854-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bancks MP, Carson AP, Lewis CE, Gunderson EP, Reis JP, Schreiner PJ, Yano Y, Carnethon MR. Fasting glucose variability in young adulthood and incident diabetes, cardiovascular disease and all-cause mortality. Diabetologia. 2019;62(8):1366–1374. doi: 10.1007/s00125-019-4901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu JH, Han K, Park S, Lee DY, Nam GE, Seo JA, Kim SG, Baik SH, Park YG, Kim SM, et al. Effects of long-term glycemic variability on incident cardiovascular disease and mortality in subjects without diabetes: a nationwide population-based study. Medicine. 2019;98(29):e16317. doi: 10.1097/MD.0000000000016317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takao T, Matsuyama Y, Suka M, Yanagisawa H, Iwamoto Y. The combined effect of visit-to-visit variability in HbA1c and systolic blood pressure on the incidence of cardiovascular events in patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2015;3(1):e000129. doi: 10.1136/bmjdrc-2015-000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mo Y, Zhou J, Ma X, Zhu W, Zhang L, Li J, Lu J, Hu C, Bao Y, Jia W. Haemoglobin A1c variability as an independent correlate of atherosclerosis and cardiovascular disease in Chinese type 2 diabetes. Diab Vasc Dis Res. 2018;15(5):402–408. doi: 10.1177/1479164118778850. [DOI] [PubMed] [Google Scholar]
- 48.Gu J, Fan YQ, Zhang JF, Wang CQ. Association of hemoglobin A1c variability and the incidence of heart failure with preserved ejection fraction in patients with type 2 diabetes mellitus and arterial hypertension. Hellenic J Cardiol. 2018;59(2):91–97. doi: 10.1016/j.hjc.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Gu J, Pan JA, Fan YQ, Zhang HL, Zhang JF, Wang CQ. Prognostic impact of HbA1c variability on long-term outcomes in patients with heart failure and type 2 diabetes mellitus. Cardiovasc Diabetol. 2018;17(1):96. doi: 10.1186/s12933-018-0739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yokota S, Tanaka H, Mochizuki Y, Soga F, Yamashita K, Tanaka Y, Shono A, Suzuki M, Sumimoto K, Mukai J, et al. Association of glycemic variability with left ventricular diastolic function in type 2 diabetes mellitus. Cardiovasc Diabetol. 2019;18(1):166. doi: 10.1186/s12933-019-0971-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akaza M, Akaza I, Kanouchi T, Sasano T, Sumi Y, Yokota T. Nerve conduction study of the association between glycemic variability and diabetes neuropathy. Diabetol Metab Synd. 2018;10:69. doi: 10.1186/s13098-018-0371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pai YW, Lin CH, Lee IT, Chang MH. Variability of fasting plasma glucose and the risk of painful diabetic peripheral neuropathy in patients with type 2 diabetes. Diabetes Metab. 2018;44(2):129–134. doi: 10.1016/j.diabet.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 53.Yang CP, Li CI, Liu CS, Lin WY, Hwang KL, Yang SY, Li TC, Lin CC. Variability of fasting plasma glucose increased risks of diabetic polyneuropathy in T2DM. Neurology. 2017;88(10):944–951. doi: 10.1212/WNL.0000000000003682. [DOI] [PubMed] [Google Scholar]
- 54.Su JB, Zhao LH, Zhang XL, Cai HL, Huang HY, Xu F, Chen T, Wang XQ. HbA1c variability and diabetic peripheral neuropathy in type 2 diabetic patients. Cardiovasc Diabetol. 2018;17(1):47. doi: 10.1186/s12933-018-0693-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosa L, Zajdenverg L, Souto DL, Dantas JR, Pinto MVR, Salles G, Rodacki M. HbA1c variability and long-term glycemic control are linked to diabetic retinopathy and glomerular filtration rate in patients with type 1 diabetes and multiethnic background. J Diabetes Compl. 2019;33(9):610–615. doi: 10.1016/j.jdiacomp.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 56.Lai YR, Chiu WC, Huang CC, Tsai NW, Wang HC, Lin WC, Cheng BC, Su YJ, Su CM, Hsiao SY, et al. HbA1C Variability is strongly associated with the severity of peripheral neuropathy in patients with type 2 diabetes. Front Neurosci. 2019;13:90. doi: 10.3389/fnins.2019.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lai YR, Huang CC, Chiu WC, Liu RT, Tsai NW, Wang HC, Lin WC, Cheng BC, Su YJ, Su CM, et al. HbA1C variability is strongly associated with the severity of cardiovascular autonomic neuropathy in patients with type 2 diabetes after longer diabetes duration. Front Neurosci. 2019;13:458. doi: 10.3389/fnins.2019.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schreur V, van Asten F, Ng H, Weeda J, Groenewoud JMM, Tack CJ, Hoyng CB, de Jong EK, Klaver CCW, Jeroen Klevering B. Risk factors for development and progression of diabetic retinopathy in Dutch patients with type 1 diabetes mellitus. Acta Ophthalmol. 2018;96(5):459–464. doi: 10.1111/aos.13815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao Q, Zhou F, Zhang Y, Zhou X, Ying C. Fasting plasma glucose variability levels and risk of adverse outcomes among patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2019;148:23–31. doi: 10.1016/j.diabres.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 60.Song KH, Jeong JS, Kim MK, Kwon HS, Baek KH, Ko SH, Ahn YB. Discordance in risk factors for the progression of diabetic retinopathy and diabetic nephropathy in patients with type 2 diabetes mellitus. J Diab Invest. 2019;10(3):745–752. doi: 10.1111/jdi.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ceriello A, De Cosmo S, Rossi MC, Lucisano G, Genovese S, Pontremoli R, Fioretto P, Giorda C, Pacilli A, Viazzi F, et al. Variability in HbA1c, blood pressure, lipid parameters and serum uric acid, and risk of development of chronic kidney disease in type 2 diabetes. Diabetes Obes Metab. 2017;19(11):1570–1578. doi: 10.1111/dom.12976. [DOI] [PubMed] [Google Scholar]
- 62.Lee CL, Chen CH, Wu MJ, Tsai SF. The variability of glycated hemoglobin is associated with renal function decline in patients with type 2 diabetes. Ther Adv Chronic Dis. 2020;11:2040622319898370. doi: 10.1177/2040622319898370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Viazzi F, Russo GT, Ceriello A, Fioretto P, Giorda C, De Cosmo S, Pontremoli R. Natural history and risk factors for diabetic kidney disease in patients with T2D: lessons from the AMD-annals. J Nephrol. 2019;32(4):517–525. doi: 10.1007/s40620-018-00561-3. [DOI] [PubMed] [Google Scholar]
- 64.Lee MY, Huang JC, Chen SC, Chiou HC, Wu PY. Association of HbA1C variability and renal progression in patients with type 2 diabetes with chronic kidney disease stages 3(-)4. Int J Mol Sci. 2018;19:12. doi: 10.3390/ijms19124116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rama Chandran S, Tay WL, Lye WK, Lim LL, Ratnasingam J, Tan ATB, Gardner DSL. Beyond HbA1c: comparing glycemic variability and glycemic indices in predicting hypoglycemia in type 1 and type 2 diabetes. Diab Technol Ther. 2018;20(5):353–362. doi: 10.1089/dia.2017.0388. [DOI] [PubMed] [Google Scholar]
- 66.Gomez AM, Munoz OM, Marin A, Fonseca MC, Rondon M, Robledo Gomez MA, Sanko A, Lujan D, Garcia-Jaramillo M, Leon Vargas FM. Different indexes of glycemic variability as identifiers of patients with risk of hypoglycemia in type 2 diabetes mellitus. J Diabetes Sci Technol. 2018;12(5):1007–1015. doi: 10.1177/1932296818758105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gomez AM, Henao DC, Imitola Madero A, Taboada LB, Cruz V, Robledo Gomez MA, Rondon M, Munoz-Velandia O, Garcia-Jaramillo M, Leon Vargas FM. Defining high glycemic variability in type 1 diabetes: comparison of multiple indexes to identify patients at risk of hypoglycemia. Diabetes Technol Ther. 2019;21(8):430–439. doi: 10.1089/dia.2019.0075. [DOI] [PubMed] [Google Scholar]
- 68.DeVries JH, Bailey TS, Bhargava A, Gerety G, Gumprecht J, Heller S, Lane W, Wysham CH, Zinman B, Bak BA, et al. Day-to-day fasting self-monitored blood glucose variability is associated with risk of hypoglycaemia in insulin-treated patients with type 1 and type 2 diabetes: a post hoc analysis of the SWITCH Trials. Diabetes Obes Metab. 2019;21(3):622–630. doi: 10.1111/dom.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klimontov VV, Myakina NE. Glucose variability indices predict the episodes of nocturnal hypoglycemia in elderly type 2 diabetic patients treated with insulin. Diabetes Metab Syndr. 2017;11(2):119–124. doi: 10.1016/j.dsx.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 70.Uemura F, Okada Y, Torimoto K, Tanaka Y. Relation between hypoglycemia and glycemic variability in type 2 diabetes patients with insulin therapy: a study based on continuous glucose monitoring. Diabetes Technol Ther. 2018;20(2):140–146. doi: 10.1089/dia.2017.0306. [DOI] [PubMed] [Google Scholar]
- 71.Zhong VW, Juhaeri J, Cole SR, Shay CM, Gordon-Larsen P, Kontopantelis E, Mayer-Davis EJ. HbA1C variability and hypoglycemia hospitalization in adults with type 1 and type 2 diabetes: a nested case-control study. J Diabetes Complications. 2018;32(2):203–209. doi: 10.1016/j.jdiacomp.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 72.Lanspa MJ, Dickerson J, Morris AH, Orme JF, Holmen J, Hirshberg EL. Coefficient of glucose variation is independently associated with mortality in critically ill patients receiving intravenous insulin. Crit Care (London, England) 2014;18(2):R86. doi: 10.1186/cc13851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Timmons JG, Cunningham SG, Sainsbury CA, Jones GC. Inpatient glycemic variability and long-term mortality in hospitalized patients with type 2 diabetes. J Diabetes Complications. 2017;31(2):479–482. doi: 10.1016/j.jdiacomp.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 74.Lee CL, Sheu WH, Lee IT, Lin SY, Liang WM, Wang JS, Li YF. Trajectories of fasting plasma glucose variability and mortality in type 2 diabetes. Diabetes Metab. 2018;44(2):121–128. doi: 10.1016/j.diabet.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 75.Orsi E, Solini A, Bonora E, Fondelli C, Trevisan R, Vedovato M, Cavalot F, Gruden G, Morano S, Nicolucci A, et al. Haemoglobin A1c variability is a strong, independent predictor of all-cause mortality in patients with type 2 diabetes. Diabetes Obes Metab. 2018;20(8):1885–1893. doi: 10.1111/dom.13306. [DOI] [PubMed] [Google Scholar]
- 76.Xu D, Fang H, Xu W, Yan Y, Liu Y, Yao B. Fasting plasma glucose variability and all-cause mortality among type 2 diabetes patients: a dynamic cohort study in Shanghai, China. Sci Rep. 2016;6:39633. doi: 10.1038/srep39633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Echouffo-Tcheugui JB, Zhao S, Brock G, Matsouaka RA, Kline D, Joseph JJ. Visit-to-visit glycemic variability and risks of cardiovascular events and all-cause mortality: the ALLHAT Study. Diabetes Care. 2019;42(3):486–493. doi: 10.2337/dc18-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Akirov A, Diker-Cohen T, Masri-Iraqi H, Shimon I. High glucose variability increases mortality risk in hospitalized patients. J Clin Endocrinol Metab. 2017;102(7):2230–2241. doi: 10.1210/jc.2017-00450. [DOI] [PubMed] [Google Scholar]
- 79.Walker GS, Cunningham SG, Sainsbury CAR, Jones GC. HbA1c variability is associated with increased mortality and earlier hospital admission in people with Type 1 diabetes. Diab Med. 2017;34(11):1541–1545. doi: 10.1111/dme.13455. [DOI] [PubMed] [Google Scholar]
- 80.Critchley JA, Carey IM, Harris T, DeWilde S, Cook DG. Variability in glycated hemoglobin and risk of poor outcomes among people with type 2 diabetes in a large primary care cohort study. Diabetes Care. 2019;42(12):2237–2246. doi: 10.2337/dc19-0848. [DOI] [PubMed] [Google Scholar]
- 81.Sheng CS, Tian J, Miao Y, Cheng Y, Yang Y, Reaven PD, Bloomgarden ZT, Ning G. Prognostic significance of long-term HbA 1c variability for all-cause mortality in the ACCORD Trial. Diabetes Care. 2020;43(6):1185–1190. doi: 10.2337/dc19-2589. [DOI] [PubMed] [Google Scholar]
- 82.Forbes A, Murrells T, Mulnier H, Sinclair AJ. Mean HbA1c, HbA1c variability, and mortality in people with diabetes aged 70 years and older: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6(6):476–486. doi: 10.1016/S2213-8587(18)30048-2. [DOI] [PubMed] [Google Scholar]
- 83.Ravona-Springer R, Heymann A, Schmeidler J, Moshier E, Guerrero-Berroa E, Soleimani L, Sano M, Leroith D, Preiss R, Tzukran R, et al. Hemoglobin A1c variability predicts symptoms of depression in elderly individuals with type 2 diabetes. Diabetes Care. 2017;40(9):1187–1193. doi: 10.2337/dc16-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li TC, Yang CP, Tseng ST, Li CI, Liu CS, Lin WY, Hwang KL, Yang SY, Chiang JH, Lin CC. Visit-to-visit variations in fasting plasma glucose and HbA1c Associated With an Increased Risk of Alzheimer Disease: Taiwan Diabetes Study. Diabetes Care. 2017;40(9):1210–1217. doi: 10.2337/dc16-2238. [DOI] [PubMed] [Google Scholar]
- 85.Bancks MP, Carnethon MR, Jacobs DR, Jr, Launer LJ, Reis JP, Schreiner PJ, Shah RV, Sidney S, Yaffe K, Yano Y, et al. Fasting glucose variability in young adulthood and cognitive function in middle age: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetes Care. 2018;41(12):2579–2585. doi: 10.2337/dc18-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saito Y, Noto H, Takahashi O, Kobayashi D. Visit-to-visit hemoglobin A1c variability is associated with later cancer development in patients with diabetes mellitus. Cancer J (Sudbury, Mass) 2019;25(4):237–240. doi: 10.1097/PPO.0000000000000387. [DOI] [PubMed] [Google Scholar]
- 87.Breton MD, Patek SD, Lv D, Schertz E, Robic J, Pinnata J, Kollar L, Barnett C, Wakeman C, Oliveri M, et al. Continuous glucose monitoring and insulin informed advisory system with automated titration and dosing of insulin reduces glucose variability in type 1 diabetes mellitus. Diabetes Technol Ther. 2018;20(8):531–540. doi: 10.1089/dia.2018.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Volcansek S, Lunder M, Janez A. Acceptability of continuous glucose monitoring in elderly diabetes patients using multiple daily insulin injections. Diabetes Technol Ther. 2019;21(10):566–574. doi: 10.1089/dia.2019.0131. [DOI] [PubMed] [Google Scholar]
- 89.Avari P, Moscardo V, Jugnee N, Oliver N, Reddy M. Glycemic variability and hypoglycemic excursions with continuous glucose monitoring compared to intermittently scanned continuous glucose monitoring in adults with highest risk type 1 diabetes. J Diabetes Sci Technol. 2019 doi: 10.1177/1932296819867688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deiss D, Szadkowska A, Gordon D, Mallipedhi A, Schutz-Fuhrmann I, Aguilera E, Ringsell C, De Block C, Irace C. Clinical practice recommendations on the routine use of eversense, the first long-term implantable continuous glucose monitoring system. Diabetes Technol Ther. 2019;21(5):254–264. doi: 10.1089/dia.2018.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iga R, Uchino H, Kanazawa K, Usui S, Miyagi M, Kumashiro N, Yoshino H, Ando Y, Hirose T. Glycemic variability in type 1 diabetes compared with Degludec and Glargine on the morning injection: an open-label randomized controlled trial. Diabetes Ther. 2017;8(4):783–792. doi: 10.1007/s13300-017-0269-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rodbard HW, Peters AL, Slee A, Cao A, Traina SB, Alba M. The Effect of Canagliflozin, a sodium glucose cotransporter 2 inhibitor, on glycemic end points assessed by continuous glucose monitoring and patient-reported outcomes among people with type 1 diabetes. Diabetes Care. 2017;40(2):171–180. doi: 10.2337/dc16-1353. [DOI] [PubMed] [Google Scholar]
- 93.Mathieu C, Dandona P, Phillip M, Oron T, Lind M, Hansen L, Thoren F, Xu J, Langkilde AM. Glucose variables in type 1 diabetes studies with dapagliflozin: pooled analysis of continuous glucose monitoring data from DEPICT-1 and -2. Diabetes Care. 2019;42(6):1081–1087. doi: 10.2337/dc18-1983. [DOI] [PubMed] [Google Scholar]
- 94.Henry RR, Strange P, Zhou R, Pettus J, Shi L, Zhuplatov SB, Mansfield T, Klein D, Katz A. Effects of dapagliflozin on 24-hour glycemic control in patients with type 2 diabetes: a randomized controlled trial. Diabetes Technol Ther. 2018;20(11):715–724. doi: 10.1089/dia.2018.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nishimura R, Osonoi T, Koike Y, Miyata K, Shimasaki Y. A randomized pilot study of the effect of trelagliptin and alogliptin on glycemic variability in patients with type 2 diabetes. Adv Ther. 2019;36(11):3096–3109. doi: 10.1007/s12325-019-01097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bajaj HS, Venn K, Ye C, Patrick A, Kalra S, Khandwala H, Aslam N, Twum-Barima D, Aronson R. Lowest glucose variability and hypoglycemia are observed with the combination of a GLP-1 receptor agonist and basal insulin (VARIATION Study) Diabetes Care. 2017;40(2):194–200. doi: 10.2337/dc16-1582. [DOI] [PubMed] [Google Scholar]
- 97.Frias JP, Nakhle S, Ruggles JA, Zhuplatov S, Klein E, Zhou R, Strange P. Exenatide once weekly improved 24-hour glucose control and reduced glycaemic variability in metformin-treated participants with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2017;19(1):40–48. doi: 10.1111/dom.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Umpierrez GE, O’Neal D, DiGenio A, Goldenberg R, Hernandez-Triana E, Lin J, Park CY, Renard E, Kovatchev B. Lixisenatide reduces glycaemic variability in insulin-treated patients with type 2 diabetes. Diabetes Obes Metab. 2017;19(9):1317–1321. doi: 10.1111/dom.12930. [DOI] [PubMed] [Google Scholar]
- 99.Sofizadeh S, Imberg H, Olafsdottir AF, Ekelund M, Dahlqvist S, Hirsch I, Filipsson K, Ahren B, Sjoberg S, Tuomilehto J, et al. Effect of liraglutide on times in glycaemic ranges as assessed by CGM for type 2 diabetes patients treated with multiple daily insulin injections. Diabetes Ther. 2019;10(6):2115–2130. doi: 10.1007/s13300-019-00692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Park SE, Lee BW, Kim JH, Lee WJ, Cho JH, Jung CH, Lee SH, Suh S, Hur GC, Kim SH, et al. Effect of gemigliptin on glycaemic variability in patients with type 2 diabetes (STABLE study) Diabetes Obes Metab. 2017;19(6):892–896. doi: 10.1111/dom.12869. [DOI] [PubMed] [Google Scholar]
- 101.Kim NH, Kim DL, Kim KJ, Kim NH, Choi KM, Baik SH, Kim SG. Effects of vildagliptin or pioglitazone on glycemic variability and oxidative stress in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a 16-week, randomised, open label, Pilot Study. Endocrinol Metab (Seoul, Korea) 2017;32(2):241–247. doi: 10.3803/EnM.2017.32.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim G, Oh S, Jin SM, Hur KY, Kim JH, Lee MK. The efficacy and safety of adding either vildagliptin or glimepiride to ongoing metformin therapy in patients with type 2 diabetes mellitus. Expert Opin Pharmacother. 2017;18(12):1179–1186. doi: 10.1080/14656566.2017.1353080. [DOI] [PubMed] [Google Scholar]
- 103.Famulla S, Pieber TR, Eilbracht J, Neubacher D, Soleymanlou N, Woerle HJ, Broedl UC, Kaspers S. Glucose exposure and variability with empagliflozin as adjunct to insulin in patients with type 1 diabetes: continuous glucose monitoring data from a 4-week, randomized, Placebo-Controlled Trial (EASE-1) Diabetes Technol Ther. 2017;19(1):49–60. doi: 10.1089/dia.2016.0261. [DOI] [PubMed] [Google Scholar]
- 104.Suzuki D, Yamada H, Yoshida M, Funazaki S, Amamoto M, Morimoto J, Hara K. Sodium-glucose cotransporter 2 inhibitors improved time-in-range without increasing hypoglycemia in Japanese patients with type 1 diabetes: a retrospective, single-center, pilot study. J Diabetes Invest. 2020. [DOI] [PMC free article] [PubMed]
- 105.Zhang Y, Zhao Z, Wang S, Zhu W, Jiang Y, Sun S, Chen C, Wang K, Mu L, Cao J, et al. Intensive insulin therapy combined with metformin is associated with reduction in both glucose variability and nocturnal hypoglycaemia in patients with type 2 diabetes. Diabetes Metab Res Rev. 2017;33:7. doi: 10.1002/dmrr.2913. [DOI] [PubMed] [Google Scholar]
- 106.Mori Y, Ohta T, Yokoyama J, Utsunomiya K. Effects of low-carbohydrate/high-monounsaturated fatty acid liquid diets on diurnal glucose variability and insulin dose in type 2 diabetes patients on tube feeding who require insulin therapy. Diabetes Technol Ther. 2013;15(9):762–767. doi: 10.1089/dia.2013.0066. [DOI] [PubMed] [Google Scholar]
- 107.Blaychfeld-Magnazi M, Reshef N, Zornitzki T, Madar Z, Knobler H. The effect of a low-carbohydrate high-fat diet and ethnicity on daily glucose profile in type 2 diabetes determined by continuous glucose monitoring. Eur J Nutr. 2019 doi: 10.1007/s00394-019-02043-z. [DOI] [PubMed] [Google Scholar]
- 108.Ranjan A, Schmidt S, Damm-Frydenberg C, Holst JJ, Madsbad S, Norgaard K. Short-term effects of a low carbohydrate diet on glycaemic variables and cardiovascular risk markers in patients with type 1 diabetes: a randomized open-label crossover trial. Diabetes Obes Metab. 2017;19(10):1479–1484. doi: 10.1111/dom.12953. [DOI] [PubMed] [Google Scholar]
- 109.Ahola AJ, Forsblom C, Harjutsalo V, Groop PH. Dietary carbohydrate intake and cardio-metabolic risk factors in type 1 diabetes. Diabetes Res Clin Pract. 2019;155:107818. doi: 10.1016/j.diabres.2019.107818. [DOI] [PubMed] [Google Scholar]
- 110.Eiswirth M, Clark E, Diamond M. Low carbohydrate diet and improved glycaemic control in a patient with type one diabetes. Endocrinol Diabetes Metab Case Rep. 2018;2018:18. doi: 10.1530/EDM-18-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chang CR, Francois ME, Little JP. Restricting carbohydrates at breakfast is sufficient to reduce 24-hour exposure to postprandial hyperglycemia and improve glycemic variability. Am J Clin Nutr. 2019;109(5):1302–1309. doi: 10.1093/ajcn/nqy261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Henry CJ, Kaur B, Quek RYC, Camps SG. A low glycaemic index diet incorporating isomaltulose is associated with lower glycaemic response and variability, and promotes fat oxidation in Asians. Nutrients. 2017;9:5. doi: 10.3390/nu9050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Camps SG, Kaur B, Quek RYC, Henry CJ. Does the ingestion of a 24 hour low glycaemic index Asian mixed meal diet improve glycaemic response and promote fat oxidation? A controlled, randomized cross-over study. Nutr J. 2017;16(1):43. doi: 10.1186/s12937-017-0258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shukla AP, Dickison M, Coughlin N, Karan A, Mauer E, Truong W, Casper A, Emiliano AB, Kumar RB, Saunders KH, et al. The impact of food order on postprandial glycaemic excursions in prediabetes. Diabetes Obes Metab. 2019;21(2):377–381. doi: 10.1111/dom.13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Trico D, Filice E, Trifiro S, Natali A. Manipulating the sequence of food ingestion improves glycemic control in type 2 diabetic patients under free-living conditions. Nutr Diabetes. 2016;6(8):e226. doi: 10.1038/nutd.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Figueira FR, Umpierre D, Casali KR, Tetelbom PS, Henn NT, Ribeiro JP, Schaan BD. Aerobic and combined exercise sessions reduce glucose variability in type 2 diabetes: crossover randomized trial. PLoS ONE. 2013;8(3):e57733. doi: 10.1371/journal.pone.0057733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Farabi SS, Carley DW, Smith D, Quinn L. Impact of exercise on diurnal and nocturnal markers of glycaemic variability and oxidative stress in obese individuals with type 2 diabetes or impaired glucose tolerance. Diabetes Vasc Dis Res. 2015;12(5):381–385. doi: 10.1177/1479164115579003. [DOI] [PubMed] [Google Scholar]
- 118.Schein A, Correa A, Casali KR, Schaan BD. Are glucose levels, glucose variability and autonomic control influenced by inspiratory muscle exercise in patients with type 2 diabetes? Study protocol for a randomized controlled trial. Trials. 2016;17:38. doi: 10.1186/s13063-016-1156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Karstoft K, Clark MA, Jakobsen I, Muller IA, Pedersen BK, Solomon TP, Ried-Larsen M. The effects of 2 weeks of interval vs continuous walking training on glycaemic control and whole-body oxidative stress in individuals with type 2 diabetes: a controlled, randomised, crossover trial. Diabetologia. 2017;60(3):508–517. doi: 10.1007/s00125-016-4170-6. [DOI] [PubMed] [Google Scholar]
- 120.Paing AC, McMillan KA, Kirk AF, Collier A, Hewitt A, Chastin SFM. Dose-response between frequency of interruption of sedentary time and fasting glucose, the dawn phenomenon and night-time glucose in Type 2 diabetes. Diabetic Med. 2019;36(3):376–382. doi: 10.1111/dme.13829. [DOI] [PubMed] [Google Scholar]
- 121.Rafiei H, Robinson E, Barry J, Jung ME, Little JP. Short-term exercise training reduces glycaemic variability and lowers circulating endothelial microparticles in overweight and obese women at elevated risk of type 2 diabetes. Eur J Sport Sci. 2019;19(8):1140–1149. doi: 10.1080/17461391.2019.1576772. [DOI] [PubMed] [Google Scholar]
- 122.Nygaard H, Ronnestad BR, Hammarstrom D, Holmboe-Ottesen G, Hostmark AT. Effects of exercise in the fasted and postprandial state on interstitial glucose in hyperglycemic individuals. J Sport Sci Med. 2017;16(2):254–263. [PMC free article] [PubMed] [Google Scholar]
- 123.Figueira FR, Umpierre D, Bock PM, Waclawovsky G, Guerra AP, Donelli A, Andrades M, Casali KR, Schaan BD. Effect of exercise on glucose variability in healthy subjects: randomized crossover trial. Biol Sport. 2019;36(2):141–148. doi: 10.5114/biolsport.2019.83006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Solomon TPJ, Tarry E, Hudson CO, Fitt AI, Laye MJ. Immediate post-breakfast physical activity improves interstitial postprandial glycemia: a comparison of different activity-meal timings. Pflugers Arch. 2020;472(2):271–280. doi: 10.1007/s00424-019-02300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Martin CT, Criego AB, Carlson AL, Bergenstal RM. Advanced technology in the management of diabetes: which comes first-continuous glucose monitor or insulin pump? Curr DiabRep. 2019;19(8):50. doi: 10.1007/s11892-019-1177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kovatchev B, Cobelli C. Glucose variability: timing, risk analysis, and relationship to hypoglycemia in diabetes. Diabetes Care. 2016;39(4):502–510. doi: 10.2337/dc15-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chico A, Aguilera E, Ampudia-Blasco FJ, Bellido V, Cardona-Hernandez R, Escalada FJ, Fernandez D, Gomez-Peralta F, Villar N, Gorgojo JJ, et al. Clinical approach to flash glucose monitoring: an expert recommendation. J Diabetes Sci Technol. 2020;14(1):155–164. doi: 10.1177/1932296819841911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gomez-Peralta F, Dunn T, Landuyt K, Xu Y, Merino-Torres JF. Flash glucose monitoring reduces glycemic variability and hypoglycemia: real-world data from Spain. BMJ Open Diabetes Res Care. 2020;8:1. doi: 10.1136/bmjdrc-2019-001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Caprnda M, Mesarosova D, Ortega PF, Krahulec B, Egom E, Rodrigo L, Kruzliak P, Mozos I, Gaspar L. Glycemic variability and vascular complications in patients with type 2 diabetes mellitus. Folia Med. 2017;59(3):270–278. doi: 10.1515/folmed-2017-0048. [DOI] [PubMed] [Google Scholar]
- 130.Ohara M, Kohata Y, Nagaike H, Koshibu M, Gima H, Hiromura M, Yamamoto T, Mori Y, Hayashi T, Fukui T, et al. Association of glucose and blood pressure variability on oxidative stress in patients with type 2 diabetes mellitus and hypertension: a cross-sectional study. Diabetol Metab Syndr. 2019;11:29. doi: 10.1186/s13098-019-0425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rodrigues R, de Medeiros LA, Cunha LM, Garrote-Filho MDS, Bernardino Neto M, Jorge PT, Resende ES, Penha-Silva N. Correlations of the glycemic variability with oxidative stress and erythrocytes membrane stability in patients with type 1 diabetes under intensive treatment. Diabetes Res Clin Pract. 2018;144:153–160. doi: 10.1016/j.diabres.2018.01.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.