Fig. 2.
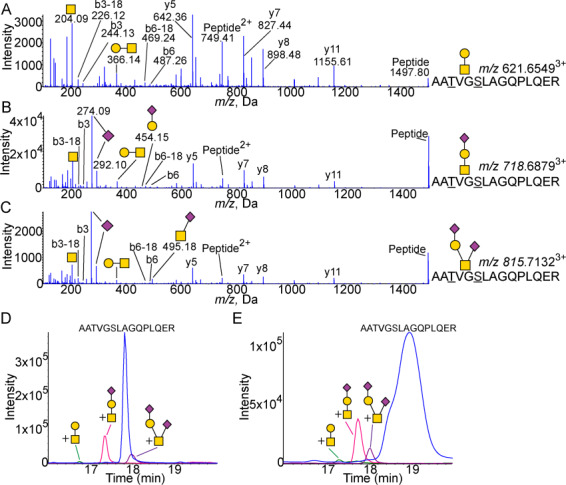
Glycosylation of the hinge domain 192–206 peptide. (A) MS/MS spectra of core 1 glycosylated AATVGSLAGQPLQER pep192–206 showing peaks Galβ1–3GalNAc (m/z 366.14) and GalNac (m/z 204.09). Expected m/z 621.6496. (B) MS/MS spectra of the peptide with Neu5Acα2–3Galβ1–3GalNAcα1 attached showing peaks of Neu5Ac (m/z 274.09 and 292.10) as well as Galβ1–3GalNAc (m/z 366.14) and GalNac (m/z 204.09). The linear structure is confirmed by the m/z 454.15 Neu5Acα2–3Gal fragment. Expected m/z 718.6813. (C) MS/MS spectra of peptide with Neu5Acα2–3Galβ1–3(Neu5Acα2–6)GalNAcα1 attached showing peaks of Neu5Ac (m/z 274.09 and 292.10) as well as Galβ1–3GalNAc (m/z 366.14), Neu5Acα2–6GalNAc (m/z 495.18) and GalNac (m/z 204.09). Expected m/z 815.7131. XICs of unglycosylated and glycosylated 192–206 peptide from (D) CSF (n = 4) and (E) plasma (n = 4). Blue is peptide. Green is peptide with core 1 structure attached. Pink is peptide with sialylated core 1 structure attached. Purple is peptide with disialylated core 1 structure attached. All masses are observed masses.
