Abstract
Background
Obstructive sleep apnea (OSA) is present in 60% to 70% of stroke patients. Cerebral vasoreactivity in patients with stroke and OSA has not been well studied and could identify a new pathophysiologic mechanism with potential therapeutic intervention. We aimed to determine whether risk categories for OSA are associated with cerebral vasoreactivity in stroke patients.
Methods and Results
In this cross‐sectional study of a cohort of patients with stroke, we used clinical questionnaires (Sleep Obstructive Apnea Score Optimized for Stroke [SOS] and snoring, tiredness, observed, pressure, bmi, age, neck, gender [STOP‐BANG] scores) to assess the risk of OSA and transcranial Doppler to assess cerebral vasoreactivity (breath‐holding index and visual evoked flow velocity response). Of the 99 patients included, 77 (78%) had medium or high risk of OSA and 80 performed transcranial Doppler. Mean breath‐holding index was 0.52±0.37, and median visual evoked flow velocity response was 10.8% (interquartile range: 8.8–14.5); 54 of 78 (69%) showed impaired anterior circulation vasoreactivity (breath‐holding index <0.69) and 53 of 71 (75%) showed impaired posterior circulation vasoreactivity (visual evoked flow velocity response ≤14.0%). There was a significant negative correlation between the risk of OSA calculated by STOP‐BANG and the breath‐holding index (rS=−0.284, P=0.012). The following variables were associated with low anterior circulation vasoreactivity: dyslipidemia (odds ratio: 4.7; 95% CI, 1.5–14.2) and STOP‐BANG score (odds ratio: 1.7 per 1‐point increase; 95% CI, 1.1–1.5).
Conclusions
A high risk of OSA and impaired vasoreactivity exists in the population that has had stroke. Dyslipidemia and STOP‐BANG sleep apnea risk categories were independently associated with impaired anterior circulation vasoreactivity.
Keywords: cerebral vasoreactivity, obstructive sleep apnea, stroke, transcranial Doppler, ultrasonography
Subject Categories: Cerebrovascular Disease/Stroke, Ischemic Stroke, Ultrasound, Metabolism
Clinical Perspective
What Is New?
A significant association exists between the risk of obstructive sleep apnea and the presence of changes in cerebral vasoreactivity in the circulation in patients with stroke.
The presence of dyslipidemia was associated with the presence of altered vasoreactivity in the anterior circulation.
What Are the Clinical Implications?
Physicians should consider screening for obstructive sleep apnea in all patients with stroke.
The causality between obstructive sleep apnea and cerebral vasoreactivity should be studied further because the decrease in vasoreactivity may be a stroke mechanism.
Nonstandard Abbreviations and Acronyms.
ACEI angiotensin converting enzyme inhibitor
ARB angiotensin receptor blocker
BHI breath‐holding index
CCB calcium channel blocker
CPAP continuous positive airway pressure
DTC transcranial Doppler
HDL high‐density lipoprotein
HRPO high‐resolution pulse oximetry
HUPES Hospital Universitário Professor Edgar Santos
LDL low‐density lipoprotein
PCA posterior cerebral artery
SOS Sleep Obstructive Apnea Score Optimized for Stroke
STOP BANG snoring, tiredness, observed, pressure, bmi, age, neck, gender
VEFVR visual evoked flow velocity response
Introduction
Stroke is a leading cause of death and incapacity in the world, affecting about 16 million people annually, of which 6 million die.1, 2 Obstructive sleep apnea (OSA) is a common condition among patients with cardiovascular and cerebrovascular events and affects 60% to 70% of this population.3, 4, 5, 6, 7 Individuals with OSA have episodes of hypoxemia, activation of the nocturnal sympathetic nervous system,8 elevation of blood pressure,9 and elevation of markers of oxidative and inflammatory stress,10 which in turn affects the cardiovascular system and contributes to the elevation of risk. The gold standard for the diagnosis of OSA is polysomnography; however, it is costly and not universally accessible.11, 12, 13 Validated clinical scores have been established as tools for the detection of risk of sleep apnea.14, 15, 16, 17
Considering that new tools may strengthen and better identify the evidence of the pathophysiologic correlation of sleep apnea and cardio‐ and cerebrovascular diseases, transcranial Doppler (TCD) can measure physiologic information and hemodynamic response at the bedside in real time.18 Cerebral vasoreactivity is defined as the response of cerebral blood vessels to changes in the partial pressure of carbon dioxide and oxygen, which can be measured by TCD through parameters such as visual evoked flow velocity response (VEFVR) and breath‐holding index (BHI).19, 20 A BHI <0.69 represents a 10‐fold increased risk of developing stroke in patients with severe stenosis or symptomatic occlusion of the internal carotid artery.21, 22, 23
The mechanisms for the development of cerebrovascular disease in OSA are not well elucidated. Patients with OSA have higher blood pressure levels and higher degrees of hypoxemia and oxidative stress, especially at night, than those without OSA, but the contribution of each of these factors is unknown.8, 9, 10 Cerebral vasoreactivity in patients with stroke and OSA has not been well studied and could identify a new pathophysiologic mechanism for potential therapeutic intervention. The purpose of this study was to determine whether risk categories for OSA are associated with cerebral vasoreactivity in stroke patients.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request. A cross‐sectional study was performed with a cohort of patients aged >18 years with stroke (both ischemic and hemorrhagic), defined as an acute focal neurologic deficit lasting >24 hours, confirmed by typical neuroimaging features on computed tomography or magnetic resonance imaging. Data collection was performed at the Magalhães Neto outpatient clinic, Professor Edgard Santos University Hospital Complex, Salvador‐Bahia, Brazil, from February 2017 to December 2018. Patients using continuous positive airway pressure for a previous diagnosis of OSA were excluded.
Patients included in the study completed a standardized questionnaire collecting data on age, sex, cerebrovascular risk factors (hypertension, dyslipidemia, diabetes mellitus, smoking history, presence of atrial fibrillation, coronary or peripheral arterial disease, heart failure), type of stroke (hemorrhagic or ischemic), and etiology of stroke (Causative Classification of Stroke System). We defined dyslipidemia as having at least one of the following measurements: total cholesterol ≥200 mg/dL, LDL (low‐density lipoprotein) ≥160 mg/dL, HDL (high‐density lipoprotein) <40 mg/dL for women or <50 mg/dL for men, triglycerides ≥150 mg/dL, use of cholesterol or triglyceride‐lowering medications.24 We also collected information of medication use and coffee consumption (number of coffee drinks per day). The risk of OSA was estimated by applying the Sleep Obstructive Apnea Score Optimized for Stroke (SOS) and snoring, tiredness, observed, pressure, bmi, age, neck, gender (STOP‐BANG) scores.14, 15, 16, 17 Patients were then referred for TCD using a 2‐MHz probe, and the evaluator was blinded to all clinical data. Patients were instructed not to drink alcoholic beverages or coffee within 24 hours before the TCD tests. The cerebral vasoreactivity indexes (VEFVR and BHI for 30 seconds) were measured, as described previously.19, 20, 25, 26, 27 Briefly, calculation of BHI was performed in the middle cerebral artery by comparing baseline velocity assessed for 30 seconds until a stable value was reached, and then measuring the highest apnea blood velocity obtained during apnea or at the first minute after apnea. In the assessment of VEFVR, which was performed at the same session as the assessment of BHI, measurements of posterior cerebral artery velocity were made with the individual keeping eyes closed for 20 seconds and were compared with the posterior cerebral artery velocity measurement taken with eyes open with a flashing light beam emitted for 40 seconds. Final VEFVR was obtained from the average of 10 measurements comparing open and closed eyes. We defined impaired anterior circulation vasoreactivity as a BHI <0.69 and impaired posterior circulation vasoreactivity as VEFVR ≤14.0%.19
Group statistics are presented as count (percentage) for categorical variables or mean±SD for continuous variables unless indicated otherwise. Deviation from normality was tested using the Kolmogorov–Smirnov test. Correlation between continuous or ordinal variables was estimated using Pearson (r p) or Spearman (r s) tests, if variables are or are not normally distributed, respectively. Univariable analyses were performed separately searching for variables associated with impaired anterior circulation vasoreactivity (BHI <0.69) or posterior circulation vasoreactivity (VEFVR ≤14.0%). For the univariable analyses, Fisher exact or Pearson χ2 tests were used for categorical variables, Student t tests were used for normally distributed continuous variables, and Mann–Whitney U tests were used for nonnormally distributed continuous variables. Those variables with a possible association in univariable tests (P<0.1) were included in multivariable logistic regression models searching separately for variables associated with impaired anterior circulation vasoreactivity or posterior circulation vasoreactivity as dependent variables. An automated backward selection process was used to reduce the number of variables in the logistic regression model, but age and sex were forced into the final model to adjust for potential confounding.
A sample of 80 patients was assessed to study association among the SOS, STOP‐ BANG, BHI, and VEFVR values, with 80% power to detect a minimum correlation index of 0.31 with α=5%. All statistical tests were 2‐tailed with a significance level of 5%. Data were analyzed using IBM SPSS v25 (IBM Corp) and visualized using GraphPad Prism v7.00 for Windows (GraphPad Software). The study was approved February 10, 2016, by the ethics committee of Professor Edgard Santos University Hospital, Federal University of Bahia, Brazil, and all patients signed informed consent.
Results
A total of 80 patients completed both clinical assessment and TCD tests (Table 1). Overall, 78 and 71 patients were able to perform the BHI and the VEFVR, respectively (2 patients had inadequate bone acoustic windows, and the velocities of the posterior cerebral arteries could not confidently be determined in 9 patients). Mean age was 57.5±13.2 years, 39 (50%) were female, 16 (21%) were smokers, and mean body mass index (kg/m2) was 26.0±4.6. The most common risk factors were hypertension in 60 (77%) and dyslipidemia in 50 (65%). Although continuous positive airway pressure was an exclusion criterion, no patients in the sample who were approached for participation were using it.
Table 1.
Univariable Analyses Comparing Stroke Patients With Normal or Abnormal Anterior Circulation Vasoreactivity
| Variable | All Patients (N=78a) | Normal Anterior Vasoreactivity (n=24) | Abnormal Anterior Vasoreactivity (n=54) | P Value |
|---|---|---|---|---|
| Age, y, mean±SD | 57±13 | 52±16 | 60±11 | 0.019 |
| Female sex, n (%) | 39 (50) | 13 (57) | 26 (47) | 0.456 |
| BMI, mean±SDa | 26±4.5 | 25.7±5.7 | 25.9±3.9 | 0.868 |
| Alcohol use, n (%) | 16 (20) | 4 (17) | 12 (22) | 0.659 |
| Smoking, n (%) | 16 (20) | 5 (22) | 11 (20) | 0.862 |
| Hypertension, n (%) | 60 (77) | 13 (56) | 47 (85) | 0.006 |
| Diabetes mellitus, n (%) | 25 (32) | 5 (22) | 20 (36) | 0.207 |
| Dyslipidemia, n (%) | 50 (64) | 8 (35) | 42 (76) | <0.001 |
| Coronary artery disease, n (%) | 9 (11) | 1 (4) | 8 (14) | 0.199 |
| Peripheral obstructive arterial disease, n (%) | 2 (3) | 0 (0) | 2 (4) | 0.354 |
| Heart failure, n (%) | 16 (20) | 4 (17) | 12 (22) | 0.659 |
| Atrial fibrillation, n (%) | 10 (13) | 4 (17) | 6 (11) | 0.435 |
| Chronic kidney disease, n (%) | 4 (5) | 1 (4) | 3 (5) | 0.840 |
| Hemorrhagic stroke, n (%) | 12 (15) | 6 (26) | 6 (11) | 0.090 |
| Vascular territory, n (%) | ||||
| Anterior circulation | 42 (78) | 10 (77) | 32 (78) | 0.932 |
| Posterior circulation | 12 (22) | 3 (23) | 9 (22) | |
| Stroke etiology, n (%) | 0.801 | |||
| Large‐vessel atherothrombosis | 2 (3) | 1 (7) | 1 (2) | |
| Small‐vessel occlusion (lacune) | 3 (5) | 0 (0) | 3 (7) | |
| Cardioembolic | 19 (32) | 6 (40) | 13 (29) | |
| Other known etiologies | 2 (3) | 0 (0) | 2 (4) | |
| Cryptogenic | 8 (14) | 2 (13) | 6 (14) | |
| >1 Cause | 1 (2) | 0 (0) | 1 (2) | |
| Incomplete investigation | 24 (41) | 6 (40) | 18 (41) | |
| Time from stroke onset to TCD, d, mean±SD | 423±174 | 428±212 | 415±158 | 0.801 |
| No. of coffee drinks per day, median (IQR) | 2 (0–4) | 2 (0–4) | 2 (0–4) | 0.965 |
| Medications in use, n (%) | ||||
| β‐Blocker | 16 (26) | 3 (13) | 13 (24) | 0.291 |
| CCB | 13 (17) | 1 (4) | 12 (22) | 0.059 |
| ACEI | 2 (3) | 0 (0) | 2 (4) | 0.354 |
| ARB | 25 (32) | 6 (26) | 19 (34) | 0.465 |
| Diuretic | 14 (18) | 2 (9) | 12 (22) | 0.168 |
| Aspirin | 19 (24) | 6 (26) | 13 (24) | 0.818 |
| Statin | 32 (41) | 11 (48) | 21 (38) | 0.430 |
| STOP‐BANG score, median (IQR) | 4 (2–5) | 2 (2–4) | 4 (3–5) | 0.002 |
| Low risk, n (%) | 24 (31) | 15 (65) | 9 (16) | <0.001 |
| Moderate risk, n (%) | 33 (42) | 4 (17) | 29 (53) | |
| High risk, n (%) | 21 (27) | 4 (17) | 17 (31) | |
| SOS score, mean±SD | 14.8±7.1 | 14.1±7.8 | 15.6±6.6 | 0.373 |
| Low risk, n (%) | 21 (27) | 9 (39) | 12 (22) | 0.207 |
| Moderate risk, n (%) | 38 (49) | 8 (35) | 30 (54) | |
| High risk, n (%) | 19 (24) | 6 (26) | 13 (25) | |
Abnormal anterior circulation vasoreactivity was defined as a breath‐holding index <0.69. SOS and STOP‐BANG are sleep apnea risk scores. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CCB, calcium channel blocker; IQR, interquartile range; and TCD, transcranial Doppler.
Two patients had insufficient bone window for insonation.
The proportion of patients considered moderate or high risk for OSA was 69% using STOP‐ BANG and 78% on the SOS. The overall mean SOS score was 14.8±7.1, and the median STOP‐BANG score was 4 (interquartile range: 2–5) among stroke patients, pointing to a general moderate to high risk for OSA. No patient underwent polysomnography for OSA diagnosis confirmation.
Cerebral Vasoreactivity
Mean BHI was 0.52±0.37, and median VEFVR was 10.8% (interquartile range: 8.8–14.5), with 54 of 78 patients (69%) showing impaired anterior circulation vasoreactivity (BHI <0.69) and 53 and 71 (75%) showing impaired posterior circulation vasoreactivity (VEFVR ≤14.0%). A moderate positive correlation was observed between the SOS and STOP‐BANG scores, with statistical significance (r s=0.5, P<0.001). A significant negative correlation (r s=−0.3, P=0.012) was observed between the STOP‐BANG and BHI scores, and a significant moderately negative correlation was observed between the SOS and VEFVR scores (r s=−0.3, P=0.008), suggesting that the higher the risk for OSA, the worse the cerebral vasoreactivity. Figure shows that patients with impaired anterior circulation vasoreactivity present with lower STOP‐BANG scores (P=0.0013).
Figure 1.
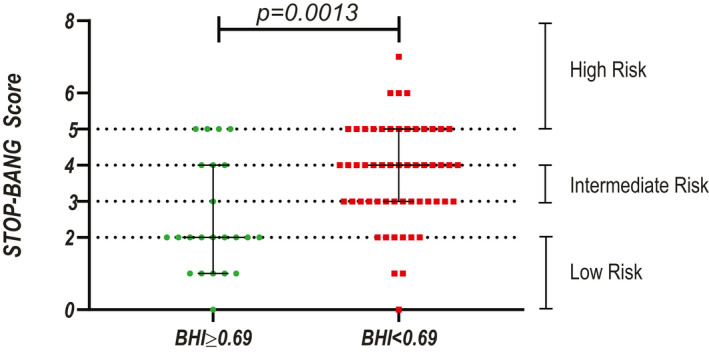
Analysis of the risk of sleep apnea (STOP‐BANG score) in stroke patients with normal or impaired anterior circulation vasoreactivity (impaired defined as breath holding index [BHI] <0.69). The black bars represent the 25th, 50th, and 75th percentiles.
Table 1 also shows a comparison of patients with normal or impaired anterior circulation vasoreactivity. Patients with impaired anterior circulation vasoreactivity were older and more likely to have a diagnosis of hypertension or dyslipidemia. Sleep apnea risk was higher among patients with impaired anterior circulation vasoreactivity, but only significantly higher when quantified by STOP‐BANG scores (median score of 2 versus 4 for normal and impaired vasoreactivity, respectively; P=0.002). There were no differences in other cerebrovascular risk factors, medication use, or coffee portions per day when comparing patients with normal and impaired anterior circulation vasoreactivity.
Posterior circulation vasoreactivity was impaired in 53 of 71 patients (75%), with a median VEFVR of 10.8% (interquartile range: 8.8–14.5). A comparison between patients with normal or impaired posterior circulation is shown in the Table 2. There were no significant differences between patients with normal or impaired posterior circulation vasoreactivity except for the use of angiotensin receptor blocker medications, which were used more frequently among patients with impaired posterior circulation vasoreactivity (Table 2).
Table 2.
Univariable Analyses Comparing Stroke Patients With Normal or Abnormal Posterior Circulation Vasoreactivity (N=71a)
| Variable | Normal Posterior Vasoreactivity (n=18) | Abnormal Posterior Vasoreactivity (n=53) | P Value |
|---|---|---|---|
| Age, y, mean±SDa | 56±12 | 58±13 | 0.574 |
| Female sex, n (%) | 8 (44) | 25 (43) | 0.841 |
| BMI, mean±SDa | 24±4.3 | 26±4.6 | 0.102 |
| Alcohol use, n (%) | 5 (29) | 10 (19) | 0.424 |
| Smoking, n (%) | 3 (17) | 13 (24) | 0.490 |
| Hypertension, n (%) | 12 (67) | 42 (79) | 0.280 |
| Diabetes mellitus, n (%) | 5 (28) | 18 (34) | 0.628 |
| Dyslipidemia, n (%) | 9 (50) | 37 (70) | 0.128 |
| Coronary artery disease, n (%) | 1 (6) | 7 (13) | 0.403 |
| Peripheral obstructive arterial disease, n (%) | 0 (0) | 2 (4) | 0.538 |
| Heart failure, n (%) | 5 (28) | 11 (21) | 0.538 |
| Atrial fibrillation, n (%) | 3 (17) | 6 (11) | 0.556 |
| Chronic kidney disease, n (%) | 0 (0) | 4 (7) | 0.230 |
| Hemorrhagic stroke, n (%) | 4 (22) | 7 (13) | 0.361 |
| Vascular territory | 0.682 | ||
| Anterior circulation | 8 (80%) | 28 (74%) | |
| Posterior circulation | 2 (20%) | 10 (26%) | |
| Ischemic stroke etiology, n (%) | 0.389 | ||
| Large‐vessel atherothrombosis | 2 (5) | 0 (0) | |
| Small‐vessel occlusion (lacune) | 1 (2) | 2 (18) | |
| Cardioembolic | 5 (45) | 13 (31) | |
| Other known etiologies | 0 (0) | 2 (5) | |
| Cryptogenic | 1 (9) | 7 (17) | |
| >1 Cause | 0 (0) | 1 (2) | |
| Incomplete investigation | 3 (27) | 16 (38) | |
| Time from stroke onset to TCD, d, mean±SD | 439±184 | 410±174 | 0.581 |
| Vasodilators | 0 (0%) | 3 (6%) | 0.302 |
| Sleep‐inducing medications | 0 (0%) | 9 (17%) | 0.061 |
| No. of coffee drinks per day, median (IQR) | 2 (0–4) | 2 (1–2) | 0.937 |
| Medications in use, n (%) | |||
| β‐Blocker | 2 (11) | 9 (17) | 0.552 |
| CCB | 2 (11) | 8 (15) | 0.675 |
| ACEI | 0 (0) | 2 (4) | 0.403 |
| ARB | 2 (11) | 22 (41) | 0.018 |
| Diuretic | 1 (6) | 12 (23) | 0.104 |
| Aspirin | 4 (22) | 12 (23) | 0.404 |
| Statin | 4 (33) | 23 (43) | 0.453 |
| SOS score, mean±SD | 13.2±5.6 | 15.8±7.1 | 0.167 |
| Low risk, n (%) | 5 (28) | 13 (24) | 0.704 |
| Moderate risk, n (%) | 10 (56) | 26 (49) | |
| High risk, n (%) | 3 (17) | 14 (26) | |
| STOP‐BANG, median (IQR) | 4 (2–5) | 4 (2–5) | 0.646 |
| Low risk, n (%) | 6 (33) | 16 (30) | 0.965 |
| Moderate risk, n (%) | 7 (39) | 21 (40) | |
| High risk, n (%) | 5 (28) | 16 (30) | |
Abnormal posterior circulation vasoreactivity defined as visual evoked flow velocity response ≤14.0%. SOS and STOP‐BANG are scores used to estimate sleep apnea risk. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CCB, calcium channel blocker; IQR, interquartile range; and TCD, transcranial Doppler.
Nine patients had insufficient bone window for insonation.
In the multivariable analysis, only dyslipidemia and STOP‐BANG risk categories remained associated with impaired anterior circulation vasoreactivity (Table 3). Dyslipidemic patients were 4.7 times more likely to present impaired anterior circulation vasoreactivity compared with patients without dyslipidemia. Similarly, increasing 1 point on the STOP‐BANG score was associated with 1.7‐fold increased odds of impaired anterior circulation vasoreactivity. Because neither STOP‐BANG nor SOS scores were associated with impaired posterior circulation vasoreactivity in the univariable analyses, we did not perform multivariable analysis for this outcome.
Table 3.
Multivariable Logistic Regression Showing Variables Associated With Impaired Anterior Circulation Vasoreactivity (Breath Holding Index <0.69)
| Variable | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Dyslipidemiaa | 4.7 if present | 1.5–14.2 | 0.007 |
| STOP‐BANG score | 1.7 per 1‐point increase | 1.1–2.5 | 0.015 |
| Age | 1.0 per increased year | 0.9–1.05 | 0.956 |
| Female sex | 1.1 | 0.3–3.7 | 0.904 |
Total cholesterol ≥200 mg/dL, LDL (low‐density lipoprotein) ≥160 mg/dL, HDL (high‐density lipoprotein) <40 mg/dL for women or <50 mg/dL for men, triglycerides ≥150 mg/dL, use of cholesterol‐ or triglyceride‐lowering medications.24
Discussion
This cross‐sectional study was conducted to assess the importance of OSA in stroke patients and its association with cerebral vasoreactivity. There were 2 main results: (1) dyslipidemia was independently associated with impaired vasoreactivity, and (2) risk of OSA was associated with impaired anterior circulation vasoreactivity. Regarding the first result, other studies have shown that statin use is associated with improved cerebral vasoreactivity compared with not using statins.28, 29, 30, 31 These authors hypothesized that statins exert an effect on the endothelium, which directly and indirectly acts on the vasodilatory response closely with vascular reactivity.28, 29, 30, 31 In our study, however, statin use was not associated with impaired vasoreactivity in univariable or multivariable analyses. The effect of statin use on vasoreactivity may be restricted to those patients who are dyslipidemic, with the highest risk of impaired vasoreactivity.
Our second main result was the association between risk of OSA and impaired anterior circulation vasoreactivity. We found a significant negative correlation between the risk of OSA based on the STOP‐BANG score and BHI, suggesting that the greater the risk of OSA, the worse cerebral vasoreactivity will be in patients with stroke, a finding confirmed in the multivariable analysis. Various studies have documented impaired cerebral perfusion during obstructive apnea events.32, 33 Impaired vasoreactivity is thought to be caused by a damaged chemoreceptor or endothelial system in the conduction of the relaxation and vasodilatation of the cerebrovascular circulation, which in turn increases cerebrovascular and cardiovascular risk.34 One study of 162 patients with Alzheimer's disease (with or without OSA) found lower BHI in the OSA group, (mean: 0.74±0.32) and BHI <0.69 was independently associated with OSA (odds ratio: 5.25; 95% CI, 2.35–11.74).35 Jointly, these findings suggest that vascular risk factors act differentially on endothelial dysfunction in patients with OSA because these individuals are more susceptible to vascular injury than those without OSA.
Other cerebrovascular risk factors were also associated with impaired anterior circulation vasoreactivity in univariable analyses, such as age and history of hypertension, but did not remain in the multivariable model. Indeed, the effects of sex on cerebral hemodynamics are complex; a study comparing pre‐ and postmenopausal women of the same age showed significantly higher BHI values in the latter group (0.81±0.10 versus 1.34±0.10; P<0.0001).36 We did not collect information on menopausal status in the present study to account for this factor. Regarding hypertension, studies of cerebral vasoreactivity in normotensive and hypertensive individuals have usually shown impaired vasoreactivity in those with hypertension,37, 38, 39 but some show no association.40, 41 The effect of hypertension on cerebrovascular reactivity probably depends on how well hypertension is controlled over time—information seldom collected in observational studies.
Few studies have evaluated posterior circulation vasoreactivity in stroke. One study evaluated posterior circulation vasoreactivity in patients with amyloid angiopathy in response to visual stimulation, based on analysis of VEFVR.19 Patients with amyloid angiopathy showed lower VEFVR compared with healthy controls (8.0% versus 17.4%; P=0.002).19 Median VEFVR was 10.8% in our study, which is closer to the range detected in patients with amyloid angiopathy. Because most patients (75%) showed abnormal posterior circulation vasoreactivity, it is possible that our study lacked power to demonstrate significant associations. Another possible explanation is the known reduction of adrenergic receptors in the posterior (vertebrobasilar) circulation compared with the carotid system, as seen in cases of posterior reversible encephalopathy,42, 43, 44 in which acute and persistent elevation of pressure levels does not reach self‐regulation to prevent brain involvement in the region of posterior brain irrigation, causing injury in this specific area.45
We used SOS and STOP‐BANG scores to estimate risk of OSA, instead of direct measurement of disease severity using polysomnography. The majority of the patients involved had scores indicating moderate or high risk, based on SOS and STOP‐BANG, which have been shown to be highly predictive of OSA.17 These data were confirmed by a meta‐analysis in which sensitivity of moderate and high STOP‐BANG categories for OSA diagnosis (using polysomnography as a gold standard) was 94% to 96%,17, 46 validated in Brazil.46 The SOS score had similar sensitivity.14 Although SOS and STOP‐BANG classify apnea risk similarly, they use different baseline variables. STOP‐BANG uses some objective data not present in the SOS score, such as neck circumference, whereas SOS uses subjective daytime sleepiness questions not present in STOP‐BANG. These differences may explain why STOP‐BANG was correlated with cerebral vasoreactivity, but SOS was not. Polysomnography is the examination of choice for diagnosis of OSA, but it is a high‐cost and difficult‐to‐access test. Studies have been developed to aggregate diagnostic and screening tools for OSA with the use of high‐resolution pulse oximetry. In these studies, oxygen desaturation measured by high‐resolution pulse oximetry predicts outcomes of acute ischemic stroke in patients with OSA.47 In addition, other home‐based screening tools have been developed and should be studied further.48
Our study was performed at a single university–based medical center. However, the cerebrovascular profile showed characteristics similar to other studies of stroke and OSA and should generalize well to other hospital‐based cohorts. In the evaluation of the risk scores for OSA, based on STOP‐BANG and SOS, the majority of our patients were moderate to high risk. This finding is similar to observations in the literature, where 60% of patients with brain and cardiovascular diseases present with OSA7 and a prevalence of sleep apnea (Apnea–Hypopnea Index ≥10/h) from 52% to 62% exists in the first 24 hours after stroke.49, 50 Although we reached our recruitment goal, losses due to inadequate bone windows or quality of measurements in the posterior cerebral artery may have led to inadequate power for some analyses, especially regarding posterior circulation vasoreactivity. Nevertheless, the study provides insights into a possible differential response in anterior and posterior circulation vasoreactivity in OSA, which should be confirmed in future studies. Finally, we did not further detail the size or location of the brain lesion found on computed tomography or magnetic resonance imaging, which could have added pathophysiologic insights to our study. However, we thought that the heterogeneity of the neuroimaging techniques from multiple hospitals that referred patients to our outpatient clinic would limit our conclusions, and we chose not to perform more detailed neuroimaging analyses.
Patients with stroke frequently present with a moderate or high risk of OSA, classified by the SOS and STOP‐BANG, and have impaired vasoreactivity on TCD measurements (BHI and VEFVR). Dyslipidemia and the risk categories for OSA, based on STOP‐BANG, were independent risk factors for impaired anterior circulation vasoreactivity.
Disclosures
None.
Acknowledgments
The authors acknowledge the Stroke Clinic, Hospital Professor Edgard Santos, Federal University of Bahia, Brazil.
(J Am Heart Assoc. 2020;9:e015313 DOI: 10.1161/JAHA.119.015313.)
For Disclosures, see page 8.
References
- 1. Website of the World Health Organization (WHO). Available at: http://www.who.int/healthinfo/global_burden_disease/en/. Accessed November 11, 2016.
- 2. Website of the Ministry of Health. Available at: http://www.brasil.gov.br/saude/2012/04/acidente-vascular-cerebral-avc. Accessed November 11, 2016.
- 3. Arzt M, Young T, Finn I, Skatrud JB, Bradley TD. Association of sleep‐disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hung J, Whitford EG, Hillman DR, Parsons RW. Association of sleep apnoea with myocardial infarction in men. Lancet. 1990;336:261–264. [DOI] [PubMed] [Google Scholar]
- 5. Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta‐analysis. J Clin Sleep Med. 2010;6:131–137. [PMC free article] [PubMed] [Google Scholar]
- 6. Worsnop CJ, Naughton MT, Barter CE, Morgan TO, Anderson AI, Pierce RJ. The prevalence of obstrutive apnea in hypertensives. Am J Respir Crit Care Med. 1998;157:111–115. [DOI] [PubMed] [Google Scholar]
- 7. Dong R, Dong Z, Liu H, Shi F, Du J. Prevalence, risk factors, outcomes, and treatment of obstructive sleep apnea in patients with cerebrovascular disease: a systematic review. J Stroke Cerebrovasc Dis. 2018;27:1471–1480. [DOI] [PubMed] [Google Scholar]
- 8. Somer VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marin JM, Augusti A, Villar I, Forner M, Nieto D, Carizzo SJ, Barbé F, Vicente E, Wei Y, Nieto FJ, et al. Association between treated and untread obstructive sleep apnea and risk of hypertension. JAMA. 2012;307:2169–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lavie L. Oxidative stress in obstructive sleep apnea and intermitente hypoxia‐revisited‐the bag ugly and good:implication to the heart and brain. Sleep Med Rev. 2015;20:27–45. [DOI] [PubMed] [Google Scholar]
- 11. Rosenberg RS, Van Hout S. The American Academy of Sleep Medicine Inter‐scorer Reliability Program: respiratory events. J Clin Sleep Med. 2014;10:447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, Mediano O, Chen R, Drager LF, Liu Z, et al; SAVE investigators and Coordinators . CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931. [DOI] [PubMed] [Google Scholar]
- 13. Caballero PEJ, Navarro RC, Martín AO, Martín TS. Cerebral hemodynamic changes in obstructive sleep apnea syndrome after contínuous positive airway pressure treatment. Sleep Breath. 2013;17:1103–1108. [DOI] [PubMed] [Google Scholar]
- 14. Camilo MR, Sander HH, Eckeli AL, Fernandes RMF, dos Santos‐Pontelli TEJ, Leite JP, Pontes‐Neto OM. SOS score: an optimized score to screen acute stroke patients for obstructive sleep apnea. Sleep Med. 2014;15:1021–1024. [DOI] [PubMed] [Google Scholar]
- 15. Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, Khajehdehi A, Shapiro CM. STOP questionnaire. Anesthesiology. 2008;108:812–821. [DOI] [PubMed] [Google Scholar]
- 16. Chung F, Abdullah HR, Liao P. STOP‐bang questionnaire a practical approach to screen for obstructive sleep apnea. Chest. 2016;149:631–638. [DOI] [PubMed] [Google Scholar]
- 17. Chung F, Subramanyam R, Liao P, Sasaki E, Shapiro C, Sun Y. High STOP‐Bang score indicates a high probability of obstructive sleep apnoea. Br J Anaesth. 2012;108:768–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–774. [DOI] [PubMed] [Google Scholar]
- 19. Smith EE, Vijayappa M, Lima F, Delgado P, Wendell L, Rosand J, Greenberg SM. Impaired visual evoked flow velocity response in cerebral amyloid angiopathy. Neurology. 2008;71:1424–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Markus HS, Harrison MJG. Estimation of cerebrovascular reactivity using transcranial Doppler, including the use of breath‐holding as the vasodilatory stimulus. Stroke. 1992;23:668–673. [DOI] [PubMed] [Google Scholar]
- 21. Webster MW, Makaroun MS, Steed DL, Smith HA, Johnson DW, Yonas H. Compromised cerebral blood flow reactivity is a predictor of stroke in patients with symptomatic carotid artery occlusive disease. J Vasc Surg. 1995;21:338–344. [DOI] [PubMed] [Google Scholar]
- 22. Vernieri F, Pasqualetti P, Matteis M, Passarelli F, Troisi E, Rossini PM, Caltagirone C, Silvestrini M. Effect of collateral blood flow and cerebral vasomotor reactivity on the outcome of carotid artery occlusion. Stroke. 2001;32:1552–1558. [DOI] [PubMed] [Google Scholar]
- 23. Vernieri F, Pasqualetti P, Passarelli F, Rossini PM, Silvestrini M. Outcome of carotid artery occlusion is predicted by cerebrovascular reactivity. Stroke. 1999;30:593–598. [DOI] [PubMed] [Google Scholar]
- 24. Xavier HT, Izar MC, Faria Neto JR, Assad MH. V Diretriz Brasileira de Dislipidemias e Prevenção da Aterosclerose. Arq Bras Cardiol. 2013;101:1–22. [DOI] [PubMed] [Google Scholar]
- 25. Rosengarten B, Huwendiek O, Kaps M. Neurovascular coupling and cerebral autoregulation can be described in terms of a control system. Ultrasound Med Biol. 2001;27:189–193. [DOI] [PubMed] [Google Scholar]
- 26. Rosengarten B, Huwendiek O, Kaps M. Neurovascular coupling in terms of a control system: validation of a second order linear system model. Ultrasound Med Biol. 2001;27:631–635. [DOI] [PubMed] [Google Scholar]
- 27. Rosengarten B, Aldinger C, Spiller A, Kaps M. Neurovascular coupling remains unaffected during normal aging. J Neuroimaging. 2003;13:43–47. [PubMed] [Google Scholar]
- 28. Sterzer P, Meintzschel F, Rösler A, Lanfermann H, Steinmetz H, Sitzer M. Pravastatin improves cerebral vasomotor reactivity in patients with subcortical small‐vessel disease. Stroke. 2001;32:2817–2820. [DOI] [PubMed] [Google Scholar]
- 29. Sander K, Hof U, Poppert H, Conrad B, Sander D. Improved cerebral vasoreactivity after statin administration in healthy adults. J Neuroimaging. 2005;15:266–270. [DOI] [PubMed] [Google Scholar]
- 30. Murakami M, Fujioka S, Hirata Y, Kuratsu JI. Low‐dose of statin treatment improves cerebrovascular reactivity in patients with ischemic stroke: single photon emission computed tomography analysis. J Stroke Cerebrovasc Dis. 2008;17:16–22. [DOI] [PubMed] [Google Scholar]
- 31. Forteza A, Romano JG, Campo‐bustillo I, Campo N, Haussen DC, Gutierrez J, Koch S. High‐dose atorvastatin enhances impaired cerebral vasomotor reactivity. J Stroke Cerebrovasc Dis. 2012;21:487–492. [DOI] [PubMed] [Google Scholar]
- 32. Balfors EM, Franklin KA. Impairment in cerebral perfusion during obstructive sleep apnea. Am J Respir Crit Care Med. 1994;150:1587–1591. [DOI] [PubMed] [Google Scholar]
- 33. Franklin KA. Cerebral haemodynamics in obstructive sleep apnoea and Cheyne‐Stokes respiration. Sleep Med Rev. 2002;6:429–441. [DOI] [PubMed] [Google Scholar]
- 34. Oz O, Tardemir S, Akgun H, Erdem M, Balikci A, Cetiz A, Yucel M, Ulas UH, Demirkaya S, Kutukcu Y, et al. Decreased cerebral vasomotor reactivity in patients with obstructive sleep apnea syndrome. Sleep Med. 2017;30:88–92. [DOI] [PubMed] [Google Scholar]
- 35. Buratti L, Viticchi G, Falsetti L, Cagnetti C, Luzzi S, Bartolini M, Provinciali L, Silvestrini M. Vascular impairment in Alzheimer's disease: the role of obstructive sleep apnea. J Alzheimers Dis. 2014;38:445–453. [DOI] [PubMed] [Google Scholar]
- 36. Matteis M, Troise E, Monaldo BC, Caltagirone C. Age and sex differences in cerebral hemodynamics. Stroke. 1998;29:963–967. [DOI] [PubMed] [Google Scholar]
- 37. Ficzere A, Valikovics A, Fulesdi B, Juhász A, Czuriga I, Csiba L. Cerebrovascular reactivity in hypertensive patients: a transcranial Doppler study. J Clin Ultrasound. 1997;25:283–289. [DOI] [PubMed] [Google Scholar]
- 38. Troisi E, Attanasio A, Matteis M, Bragoni M, Monaldo BC, Caltagirone C, Silvestrini M. Cerebral hemodynamics in young hypertensive subjects and effects of atenolol treatment. J Neurol Sci. 1998;159:115–119. [DOI] [PubMed] [Google Scholar]
- 39. Yamamoto M, Meyer JS, Sakai F, Yamaguchi F. Aging and cerebral vasodilatator responses to hypercarbia‐responses in normal aging and in persons with risk factors for stroke. Arch Neurol. 1980;37:489–496. [DOI] [PubMed] [Google Scholar]
- 40. Aizawa T, Tazaki Y, Gotoh F. Cerebral circulation in cerebrovascular diseases. World Neurol. 1961;2:635–645. [PubMed] [Google Scholar]
- 41. Tominaga S, Strandgaard S, Uemura K, Ito K, Kutsuzawa T, Lassen NA. Cerebrovascular CO reactivity in normotensive and hypertensive man. Stroke. 1976;7:507–510. [DOI] [PubMed] [Google Scholar]
- 42. Sjoberg N, Edvinsson L, Owman C. Autonomic nerves, mast cells, and amine receptors in human brain vessels. A histochemical and pharmacological study. Brain Res. 1976;115:377–393. [DOI] [PubMed] [Google Scholar]
- 43. Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500. [DOI] [PubMed] [Google Scholar]
- 44. Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14:914–925. [DOI] [PubMed] [Google Scholar]
- 45. Beausang‐linder M, Bill A. Cerebral circulation in acute arterial hypertension‐protective effects of sympathetic nervous activity. Acta Physiol Scand. 1981;111:193–199. [DOI] [PubMed] [Google Scholar]
- 46. Fonseca LB, Silveira EA, Lima NM, Rabahi MF. STOP‐Bang questionnaire: translation to Portuguese and cross‐cultural adaptation for use in Brazil. J Bras Pneumol. 2016;42:266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yaddanapudi SS, Pineda MC, Boorman DW, Bryne RE, Hing KL, Sharma S. High‐resolution pulse oximetry (HRPO): a cost‐effective tool in screening for obstructive sleep apnea (OSA) in acute stroke and predicting outcome. J Stroke Cerebrovasc Dis. 2018;27:2986–2992. [DOI] [PubMed] [Google Scholar]
- 48. Boulos MI, Colleli DR, Vaccarino SR, Kamra M, Murray BJ, Swartz RH. Using a modified version of the “STOP‐BANG” questionnaire and nocturnal oxygen desaturation to predict obstructive sleep apnea after stroke or TIA. http://ClinicalTrials.gov. 2019;56:177–183. [DOI] [PubMed] [Google Scholar]
- 49. Iranzo A, Santamaría J, Berenguer J, Sánchez M, Chamorro A. Prevalence and clinical importance of sleep apnea in the first night after. Neurology. 2002;58:911–916. [DOI] [PubMed] [Google Scholar]
- 50. Väyrynen K, Kortelainen K, Numminen H, Miettinen K, Keso A, Tenhunen M, Huhtala H, Himanen SL. Screening sleep disordered breathing in stroke unit. Sleep Disord. 2014;2014:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


