Figure 5.
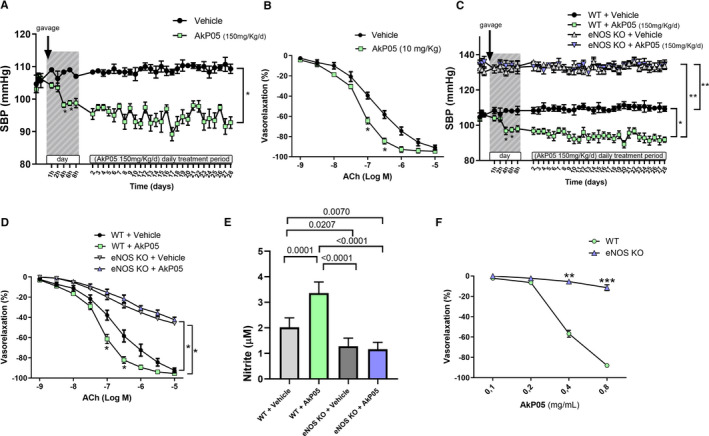
A, Time course of systolic blood pressure (SBP) measured noninvasively (A) in C57BL/6 mice receiving a daily oral dose of vehicle or AkP05 at different time‐points from first gavage administration (day 1) and after 4 hours from continued daily administration for 4 weeks. Arrow indicates start of administration via gavage. Data are mean±SEM (n=5 AkP05; n=3 Vehicle); *P<0.05 vs all. B, Dose–response curves to acetylcholine (ACh; 10−9 mol/L to 10−5 mol/L) of mesenteric arteries excised from mice at the end of treatment with of vehicle or AkP05; *P<0.05 (n=5 experiments). C, SBP in wild‐type (WT) and eNOS‐knockout (KO) mice during AkP05 or vehicle administration at different time‐points from first gavage administration (day 1) and after 4 hours from continued daily administration for 4 weeks. *P<0.05, **P<0.01; (n=5 for each group). D, Dose–response curves to acetylcholine (ACh; 10−9 mol/L to 10−5 mol/L) of mesenteric arteries obtained from wild‐type and eNOS deficient mice at the end of treatment with vehicle or AkP05; *P<0.05 (n=5 independent experiments). E, Nitrite concentration in serum collected at the end of 4‐week daily treatment in wild‐type and eNOS KO mice treated with vehicle or AkP05 (n=5). F, Dose–response curves of ex vivo mice mesenteric arteries from eNOS‐knockout mice are refractive to the vasorelaxant effect of AkP05. **P<0.01. ***P<0.001 (n=4 for each group).
