Primary prevention implantable cardioverter‐defibrillators (ICDs) play a crucial role in the treatment of patients at heightened risk of sudden cardiac death (SCD) caused by ventricular arrhythmias. The use of ICDs for primary prevention is supported by multiple, randomized clinical trials now codified into guidelines that drive clinical practice.1 However, there are well‐known shortcomings of the current paradigm, which determines primary prevention ICD candidacy based largely on left ventricular ejection fraction (LVEF) and heart failure class.2, 3 Most ICD recipients never receive appropriate device therapy, whereas others may receive shocks but die from progressive heart failure or noncardiac causes without deriving a meaningful survival benefit from the device.4 Despite well‐acknowledged limitations, there have been no significant new trials refining patient selection for primary prevention ICDs in nearly 15 years, with the guidelines themselves likely presenting a significant barrier to randomization. In this article, we review areas where current risk stratification algorithms perform poorly and highlight opportunities for improving decision making regarding ICD implantation. We also propose research and policy solutions for improving the yield of primary prevention ICD implantation.
Current Paradigm
Largely on the basis of data from the MADIT‐II (Multicenter Automatic Defibrillator Implantation Trial II),5 published in 2002, and the SCD‐HeFT (Sudden Cardiac Death in Heart Failure Trial),6 published in 2005, guidelines for ICD therapy initially published in 2008 and then updated in 2012 and 2017 recommend primary prevention device implantation principally on the basis of 2 factors: LVEF and New York Heart Association (NYHA) class.7 As these data pertain only to primary prevention ICD implantation, for the remainder of this discussion, all references to ICD implantation will be focused on primary prevention indications. Additionally, our discussion focuses only on ICD therapy for prevention of SCD, without concomitant cardiac resynchronization therapy to treat heart failure.
Since the publication of the 2008 guidelines, individuals with LVEF ≤35% who are NYHA class II or III (or class I with prior myocardial infarction and LVEF ≤30%) on optimal medical therapy and who have not had a recent acute coronary syndrome or coronary revascularization can be considered candidates for ICD implantation, with a class IA recommendation.7 However, heart failure class is notoriously subjective8 and can change significantly over time, making LVEF the main objective criterion. The guidelines also stipulate that candidates for ICD implantation should have a reasonable expectation of survival >1 year.7 Under this paradigm, which has been in place for over a decade with relatively little change, individuals with persistently impaired LVEF who are not imminently at risk of dying from other causes can be considered for ICD implantation.
On the basis of these guidelines, ≈75 000 primary prevention ICDs are implanted annually in the United States.9 But despite the large number of devices implanted, the number of patients who receive appropriate ICD therapy is relatively small. Estimates vary on the basis of specific patient factors but across most studies of primary prevention ICDs, the rate of appropriate ICD shocks is only about 5% to 10% per year.5, 6 The incidence may be even lower in contemporary practice if devices are programmed according to recent studies demonstrating the benefit of extended arrhythmia detection times and high detection rate cutoffs.10, 11, 12 Given that half of all device recipients aged >65 are dead 5 years after implantation,13 many patients who are implanted with ICDs will die before an appropriate ICD shock occurs, or shortly thereafter if the shock occurs as part of the dying process.4
Even among those with reasonable life expectancy after ICD implantation, the current paradigm is suboptimal in identifying patients who are likely to benefit. In a large national data set of over 24 000 patients undergoing replacement of ICD generators at end of battery life, only one‐third of individuals had received any ICD shocks during the first battery life. The other two‐thirds had survived through the first ICD generator (mean 5.3 years) without having received device therapy.14 These data highlight the fact that under current implantation algorithms, most individuals implanted with primary prevention ICDs receive no benefit from the device. They are, however, exposed to the risks of device implantation, generator replacements, and the potential drawbacks of living with an ICD.15, 16, 17, 18 This suggests that if risk stratification algorithms were refined, many patients could be spared from device implantation without any expected reduction in overall survival. Additionally, although it has been several years since formal cost‐effectiveness analyses of primary prevention ICD therapy have been published,19 given the large number of devices implanted annually, opportunities to refine implantation criteria hold significant promise for reducing healthcare expenditures without compromising clinical outcomes.
Why Does the Current Paradigm Perform Poorly in Predicting ICD Benefit?
Defibrillators are capable of preventing only one specific mode of death attributable to sudden arrhythmic causes. Therefore, it makes sense that the initial trials of ICD implantation used LVEF as the main inclusion criteria. LVEF is an easily measurable, quantifiable marker of SCD risk with a well‐established relationship between worsening LVEF and increased risk of arrhythmic mortality.20, 21, 22 However, although a statistically significant benefit can be demonstrated with ICD therapy in appropriately designed clinical trials that use LVEF as the main criterion for selection,5, 6 LVEF performs relatively poorly when predicting the likelihood of ICD benefit for an individual patient.
Several reasons account for the low positive predictive value of impaired LVEF in identifying patients who are likely to benefit from an ICD. First, the annual incidence of SCD is relatively low even among the highest‐risk LVEF subgroups. Among primary prevention ICD recipients, the annual risk of SCD among those with high‐risk features including prior myocardial infarction and nonsustained ventricular tachycardia is only about 10%,2, 5 suggesting that most patients would have to survive many years before expecting to derive benefit from the device. Second, low LVEF is associated not only with an increased risk of SCD but also with an increased risk of death attributable to nonarrhythmic causes, such as progressive heart failure. The fact that impaired LVEF is associated with increased risks of both sudden and nonsudden death is a major reason why using LVEF as the primary criterion for determining ICD candidacy is suboptimal. For many patients, as LVEF decreases, the risk of nonsudden death may increase at an even faster rate2, 21, 23 (Figure 1), such that the proportional risk of sudden cardiac death, which describes the ratio of sudden cardiac death to overall mortality, may actually decrease as ejection fraction worsens. Understanding this difference between absolute and proportional risk of SCD is critical to appreciating the limitations of LVEF as a predictor of ICD benefit.
Figure 1.
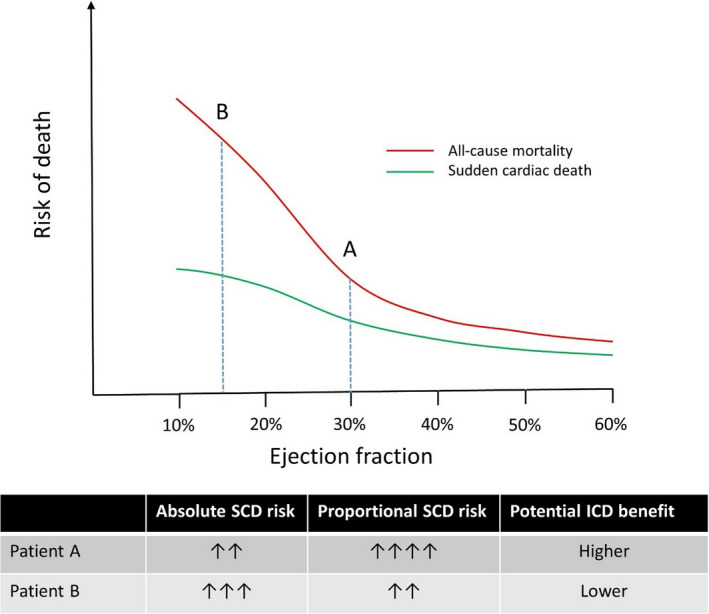
Theoretical representation of the differences in all‐cause mortality and proportional SCD risk as a function of left ventricular ejection fraction and the impact on potential defibrillator benefit. Although patient A has a higher absolute risk of SCD, the benefit to defibrillator therapy may be attenuated as a function of lower proportional sudden death risk. ICD indicates implantable cardioverter‐defibrillator; SCD, sudden cardiac death.
Taking 2 hypothetical patients with overall similar profiles except that patient A has an LVEF of 30% whereas patient B has an LVEF of 15%, the absolute risk of SCD is higher for patient B, as a function of lower ejection fraction. However, the proportional risk of SCD may be lower for patient B because of an increased risk of dying from nonarrhythmic causes, such as progressive heart failure (Figure 1). Stated another way, if patient A dies, it is far more likely that he or she will die from SCD, whereas if patient B dies, he or she may be more likely to die from nonarrhythmic causes. Even if patient A has a lower absolute risk of SCD, he or she may derive more benefit from device implantation.
It may seem counterintuitive that a patient with a lower absolute risk of SCD might derive more benefit from an ICD than another patient with a higher absolute SCD risk, but the data from seminal ICD trials suggest exactly that. In an important sub‐analysis from MADIT‐II,24 the investigators retrospectively identified clinical markers that were associated with the risk of SCD: renal function, NYHA class, age, presence of atrial fibrillation, and QRS duration. Using these variables, they classified patients enrolled in the trial into 3 groups on the basis of predicted SCD risk: low, intermediate, and high.25 At 2‐year follow‐up, a survival benefit to ICD therapy was noted only in the intermediate SCD risk group; no benefit was discernable in either the low‐ or the high‐risk groups. However, during long‐term follow‐up to 8 years, a significant survival benefit to ICD therapy also became evident in the low‐SCD‐risk group, whereas the highest‐SCD‐risk group continued to derive no benefit.24 An increased risk of death attributable to nonarrhythmic causes likely explains the lack of survival benefit to ICDs in the highest‐SCD‐risk group in MADIT‐II.
Another consideration when evaluating limitations of the current paradigm for ICD implantation is that advances in medical therapy for heart failure have had a significant impact on SCD risk. In an analysis of SCD incidence among patients enrolled in heart failure clinical trials over the past 2 decades, the annual incidence of SCD has decreased by over 40%, from ≈6% per year in the mid‐1990s to <4% per year in trials completed most recently.26 Figure 2 demonstrates the stepwise reduction in both SCD and all‐cause mortality with the addition of medical therapy for heart failure and highlights the need to account for improvements in background therapy on the potential benefit of ICDs.27 On the basis of these findings, the SCD risk profile of patients who were enrolled in the seminal ICD trials (ie, MADIT‐II and SCD‐HeFT) over a decade ago is likely different from those who are encountered in current clinical practice.
Figure 2.
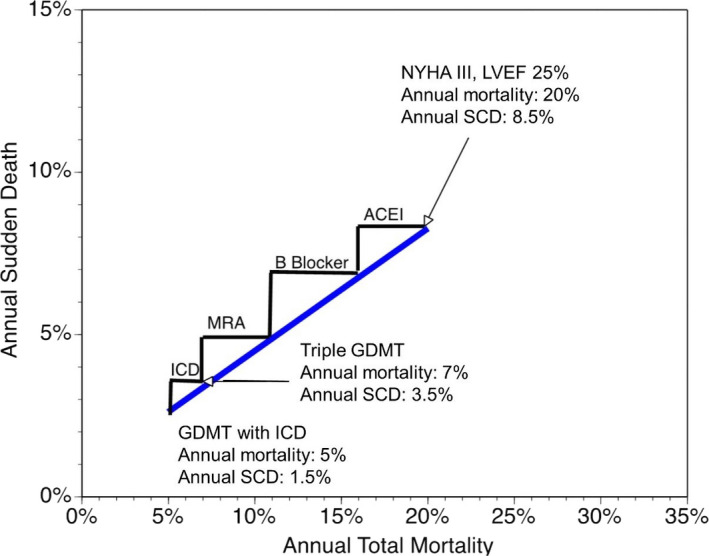
Demonstration of the stepwise reduction in all‐cause mortality and sudden cardiac death risk with guideline directed medical therapy (GDMT) for heart failure.27 ACEI indicates angiotensin‐converting enzyme inhibitor; ICD, implantable cardioverter‐defibrillator; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association Class; LVEF, left ventricle ejection fraction; SCD, sudden cardiac death.
Given the limitations of LVEF in predicting ICD benefit, considerable effort over recent years has focused on developing better tools for predicting absolute SCD risk. Many methods have been evaluated for this purpose, including measures of depolarization and repolarization dynamics, indicators of autonomic dysregulation, extent of delayed enhancement on cardiac magnetic resonance imaging, electrophysiologic testing, biomarkers, and genetics.22, 28, 29, 30, 31, 32 Some of these methods have demonstrated superiority to LVEF in predicting absolute SCD risk. However, none of these methods is likely to significantly improve prediction of ICD benefit unless it also accounts for competing risks of nonarrhythmic death.
Better Approaches to Predicting ICD Benefit
Having established the importance of evaluating competing risks in predicting ICD benefit, the next 2 questions that need to be answered are (1) at what level of proportional SCD risk is an ICD likely to be beneficial, and (2) at what level of overall mortality risk is the proportional risk of SCD so low that an ICD is no longer beneficial? To answer these questions, several important studies have evaluated ICD benefit as a function of proportional SCD risk.
The Seattle Heart Failure Model (SHFM) was initially developed to predict all‐cause mortality in patients with heart failure using commonly available clinical and demographic variables. In a paper published in 2009, the SHFM was applied to patient‐level data from SCD‐HeFT (n=2483), with patients divided into quintiles based on the SHFM‐predicted 4 year risk of all‐cause mortality.33 Several useful findings emerged from this analysis. First, the proportional risk of SCD was highest (52%) in quintiles 1 and 2, which had the lowest overall 4‐year mortality. The proportional risk of SCD was intermediate in quintiles 3 and 4 at 40% and the percentage of deaths that were adjudicated as SCD was lowest (24%) in quintile 5, which had the highest overall mortality. This finding echoes other lines of evidence that demonstrate that as the overall risk of mortality increases, the proportional risk of SCD decreases. At 4 years of follow‐up, the absolute survival benefit associated with randomization to ICD therapy in SCD‐HeFT was 6.6%, 8.8%, 10.6%, and 14.0% in quintiles 1 to 4, but minus 4.9% in quintile 5, demonstrating that ICD therapy was of no benefit among those with the highest predicted all‐cause mortality. On the basis of these findings, the investigators predicted that ICD candidates with an SHFM‐predicted 1‐year mortality of >20% to 25% are highly unlikely to benefit from ICDs, reflecting the fact that the proportional risk of SCD in this range of total mortality is too low to be meaningfully impacted by an ICD. In real‐world practice, many of these individuals with very high levels of overall mortality who are implanted with ICDs may receive appropriate ICD shocks but go on to die from other causes shortly afterwards, without deriving any meaningful survival benefit.
In a subsequent analysis, the same group of investigators developed the Seattle Proportional Risk Model (SPRM), which is designed to predict the proportional risk of SCD, independently of the SHFM‐predicted estimate of all‐cause mortality.34 In essence, the SHFM predicts the risk of all‐cause mortality, and the SPRM can then be used to predict the proportion of deaths attributable to SCD, if death occurs. Variables included in the SPRM include age, sex, diabetes mellitus, body mass index, systolic blood pressure, LVEF, NYHA class, serum sodium, creatinine, and use of digoxin—all of which are easily accessible in clinical settings and can be obtained in a highly cost‐effective manner. Some instructive patterns emerge when comparing the variables included in the SHFM and the SPRM, one of which is designed to predict all‐cause mortality, and the other is designed to predict proportional risk of SCD. As might be expected, many clinical variables were associated with increased risk of both sudden and nonsudden death. However, younger age, male sex, higher NYHA class, and higher body mass index were more strongly associated with risk of dying suddenly, whereas diabetes mellitus, renal dysfunction, hyponatremia, and systolic hyper‐ or hypotension were more strongly associated with risk of dying nonsuddenly (Figure 3). For example, the proportional risk of SCD increased by 25% for every younger decade of life, consistent with findings from clinical trials that suggest that younger patients derive more benefit from ICDs than older patients.35, 36 The derivation of these models highlights the fact that exploiting differences in clinical variables that are more closely associated with the risk of dying suddenly or nonsuddenly can be used to enrich for populations who are most likely to benefit from ICDs and withhold therapy from those who are unlikely to benefit.
Figure 3.
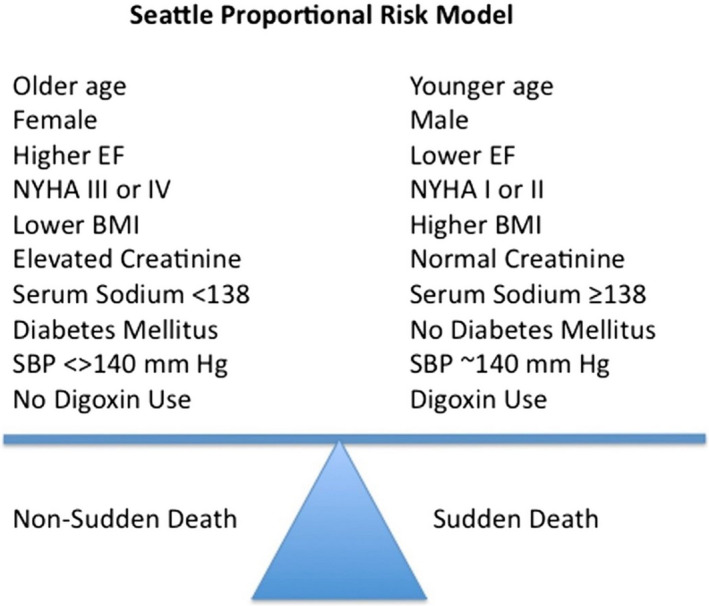
Clinical factors that are more closely associated with the risk of sudden vs nonsudden death based on the Seattle Proportional Risk Model. BMI indicates body mass index; EF, ejection fraction; NYHA, New York Heart Association; SBP, systolic blood pressure. Reproduced from Levy et al37 with permission. Copyright ©2017, Elsevier.
The SPRM was subsequently validated in a group of 2331 ambulatory patients with NYHA class II‐IV heart failure and LVEF ≤35%, of whom 1204 patients (62%) had ICDs.37 Among patients without ICDs, the SPRM was highly predictive of likelihood of dying suddenly versus nonsuddenly (odds ratio, 2.05; P=0.002). And when the SPRM was applied in the subgroup of patients with ICDs, a significant interaction was noted between the proportional risk of SCD and the magnitude of survival benefit from the device. Defibrillators had the greatest impact on survival among patients with the highest proportional risk of SCD, whereas no survival benefit was noted among those with a proportional SCD risk ≤32%.
Application of the SHFM and SPRM to patient‐level data from SCD‐HeFT demonstrated that randomization to ICD therapy was associated with a significant survival benefit among patients with low predicted overall mortality based on the SHFM and high predicted proportional SCD risk based on SPRM.38 Approximately two‐thirds of patients enrolled in SCD‐HeFT fell in this category (Figure 4), with an ≈50% reduction in all‐cause mortality from ICDs noted in this subgroup. In contrast, no significant ICD benefit was noted in the remaining one‐third of patients who had either high predicted overall mortality or low proportional SCD risk. Similar findings were noted in an analysis of over 87 000 patients with ICDs in the NCDR (National Cardiovascular Data Registry) ICD Registry, who were compared with a contemporary cohort of patients without ICDs.39 ICD therapy was most beneficial among those with low overall mortality and high proportional SCD risk, which accounted for about one‐quarter of patients implanted with ICDs in the NCDR cohort. In this subgroup, ICD treatment for only 4.2 years resulted in an overall improvement in survival of 1 year. In real‐world practice, these are the patients who benefit most from primary prevention devices. In contrast, among the group with low predicted overall mortality and low proportional SCD risk, which also accounted for about a quarter of the NCDR cohort, ICD treatment would require over 22 years to add 1 year of life. Withholding ICDs from this subgroup would be very unlikely to have a meaningful impact on overall mortality. These findings also reinforce the notion that substantial heterogeneity exists among individuals who are implanted with ICDs in current practice and that better risk stratification tools could significantly improve the yield of device implantation.
Figure 4.
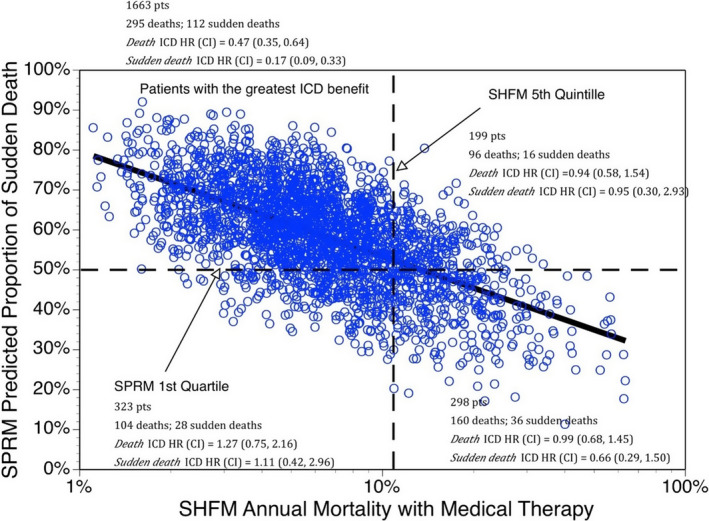
Benefit associated with randomization to implantable cardioverter‐defibrillator (ICD) therapy in the SCD‐HeFT (Sudden Cardiac Death in Heart Failure Trial). Defibrillators were associated with the greatest reduction in all‐cause mortality among patients with low overall mortality risk based on the Seattle Heart Failure Model (SHFM) and high proportional sudden cardiac death risk based on the Seattle Proportional Risk Model (SPRM). CI indicates confidence interval; HR, hazard ratio. Reprinted from Levy et al38 with permission. Copyright ©2018, Elsevier.
Although the SHFM and SPRM are among the best validated approaches to using competing risks models to predict ICD benefit, other studies have also capitalized on this idea using different approaches. Several studies have developed models in ICD recipients to predict the likelihood of dying without receiving appropriate defibrillator therapy, based on the premise that these individuals derive no benefit from their devices. Among several studies that have looked at this outcome, ≈10% to 15% of ICD recipients die without ever receiving appropriate ICD therapy.40, 41, 42 The variables that predict the highest likelihood of death without ICD therapy are similar to the variables that predict high overall mortality in the Seattle models: older age, more advanced heart failure symptoms, diabetes mellitus, and more extreme reductions in LVEF.41, 42 In effect, these differing approaches are all identifying similar sorts of patients who have high overall mortality risk and will die either before receiving ICD therapy or despite receiving ICD therapy. In either case, device implantation provides no benefit and represents an opportunity for improving patient selection.
An important and related concept to the idea of predicting high overall mortality risk and low likelihood of ICD benefit is the concept of frailty. Frailty, defined as a loss of physiological reserve and vulnerability to stressors that may span multiple physical, cognitive, and functional domains, is closely associated with high overall mortality risk.43 The Canadian Cardiovascular Society guidelines for ICD implantation specifically address frailty assessment as part of the ICD decision‐making process.44 However, additional research is needed to better understand how frailty interacts with risks of arrhythmic and nonarrhythmic death in predicting ICD benefit.
Assessing competing risks also provides useful context for understanding the results of one of the few randomized trials that has been performed in the field of primary prevention ICD implantation in recent years. The results of the DANISH (Danish Study to Assess the Efficacy of ICDs in Patients with Non‐Ischemic Systolic Heart Failure on Mortality) trial, published in 2016, suggest that patients with nonischemic cardiomyopathy may not benefit from ICDs.45 Although other explanations may account for the results of the DANISH study, including the impact of cardiac resynchronization therapy on SCD, the competing risks paradigm likely offers the most relevant perspective. It is well recognized that the absolute risk of SCD is higher in ischemic versus nonischemic cardiomyopathy.6 However, for many patients with nonischemic cardiomyopathy, the proportional risk of SCD may still be sufficiently high to benefit from an ICD, if competing risks of nonarrhythmic death are low. A subanalysis from DANISH provided support for this concept by demonstrating that patients in the trial younger than 70 years of age had a much higher proportional risk of SCD and benefited significantly from ICDs, whereas those over the age of 70 derived no benefit.36 As has already been demonstrated, older age is an important predictor of all‐cause mortality, and the findings from the DANISH subanalysis are consistent with the idea that many younger individuals with nonischemic cardiomyopathy may still benefit from ICDs, whereas the benefit is significantly attenuated above the age of 70.
The SHFM and SPRM have been applied to patients enrolled in DANISH, and the results reinforce the idea that assessment of competing risks can be used to identify which patients with nonischemic cardiomyopathy are most likely to benefit from ICDs. Among the 1116 patients enrolled in DANISH, ICD implantation in those with an SPRM score above the median was associated with a significant reduction in mortality (hazard ratio, 0.63; 95% CI, 0.43–0.94). No survival benefit to ICD implantation was noted among those with lower SPRM scores.46
In aggregate, the data on competing risks clearly demonstrate that the benefit to ICD therapy cannot be predicted accurately based only on absolute SCD risk assessment. Competing risks of nonarrhythmic death must be accounted for and models based on proportional risk of SCD are likely to provide a much more robust estimate of ICD benefit. Modeling competing risks, using commonly available clinical variables, provides the best opportunity to improve on the current paradigm for ICD implantation.
Other Opportunities for Modeling Competing Risks: ICD Generator Replacement
The discussion so far has focused on indications for de novo ICD implantation under the current paradigm. However, understanding the idea of competing risks may also provide important context for other aspects of ICD therapy. In current practice, ICD generators are often routinely replaced at the end of battery life with little assessment of whether ongoing ICD therapy is likely to be beneficial.15 All individuals are older at the time they undergo ICD generator replacement than they were at the time of initial implant, and some will have had progression of underlying cardiac and noncardiac comorbidities.9 On the basis of the evidence discussed above, it is likely that for most individuals, the risk of all‐cause mortality is higher, and the proportional risk of SCD is lower, at the time of generator replacement than it was at the time of initial ICD implantation. Among patients who did not receive shocks during the first ICD battery life, the risk of SCD after generator replacement may be even lower.14 For many of these individuals, ongoing ICD therapy may be unlikely to provide meaningful benefit. Real‐world data support the idea that overall mortality rates are higher after ICD generator replacement than after de novo device implantation47 and rates of appropriate ICD shocks are likely lower.48, 49 Developing methods for estimating patient‐specific risks of sudden and nonsudden death, and evaluating how those proportional risks change over time, may help make better decisions about who is most and least likely to benefit from ICD generator replacement.
Avoiding Unnecessary Exposure to Harm
It may be argued that trying to identify individuals who are candidates for ICDs on the basis of current guidelines, but who may not benefit because of competing risks, has the potential to unnecessarily withhold therapy from individuals who would meet inclusion criteria on the basis of clinical trials. Although this is a fair concern, the relatively small percentage of patients who receive appropriate ICD shocks and the significant expense of ICD implantation under the current paradigm19 highlight the imperative to optimize allocation. Perhaps even more importantly, for an individual patient, the decision to undergo ICD implantation is associated with risks, including a major complication rate of about 4%,50 with the highest procedural risks occurring among those who may be least likely to benefit, including the elderly18 and those with significant comorbidities.18, 50 Experiencing a major complication during ICD implantation is associated with a significantly increased risk of mortality up to 6 months after the procedure.51 Efforts to identify those who are least likely to benefit from ICD implantation may also identify those who are at the greatest risk of procedural harm.
Opportunities for Improving the Status Quo
Relatively little has changed in the approach for identifying primary prevention ICD candidates over the past decade. To a certain extent, the presence of guidelines that give strong support to implantation based on the current paradigm may hinder new developments in the field. Important opportunities exist for improving the manner in which ICDs are implanted. However, progress in the field will require acknowledgment on the part of physicians, payors, and regulators that the current paradigm for ICD implantation does not effectively identify those who are most likely to benefit, may expose many patients to procedural risks with little expectation of improved survival and results in wasteful healthcare spending. Research efforts, funding priorities, and policy decisions should be made with this perspective in mind.
Although dated, the current paradigm for ICD implantation rests on a robust body of randomized clinical trial data. However, many of the seminal trials were performed with significant support from medical device companies. It seems unlikely that similar funding will be available for studies that are designed to refine the current paradigm, particularly given the high likelihood of identifying sizable percentages of patients who are currently candidates for device therapy but may not benefit. Given these limitations, novel clinical trial formats such as those that incorporate randomization into registries or use electronic medical records for follow‐up hold substantial promise.52, 53 For instance, randomized registry trial designs have been used to test other invasive cardiovascular therapies and generate high‐level data with reduced cost and increased efficiency.54 Similar approaches may allow high‐quality, randomized trials of new ICD implantation algorithms to be performed at a fraction of the cost of the seminal ICD trials without compromising the level of evidence. The ability to monitor ICDs remotely makes this field ripe for novel trial formats that can leverage large and linked data sets to evaluate clinical outcomes with lower costs and greater speed than traditional clinical trial platforms.
However, the 2018 Centers for Medicare & Medicaid Services (CMS) coverage update for defibrillator implantation, which eliminated the requirement for participation in the NCDR ICD registry,55 represents one potentially lost opportunity in the effort to improve the current paradigm. CMS justified this decision by stating that they “believe [the registry] has served its purpose” and that “CMS believes that additional data collection is no longer needed.” Although the original requirement for registry participation was designed to answer 10 specific questions about ICD therapy in real‐world practice following the initial coverage decision in 2005, the NCDR has contributed substantially to understanding the risks and benefits of ICD therapy well beyond the 10 hypotheses initially set forth by CMS. Since the registry was originally implemented, the NCDR ICD registry can now be linked with remote device monitoring databases, billing claims data and survival data from the Social Security Death Index.13, 39, 47, 56, 57, 58 These resources have contributed substantially to our understanding of real‐world ICD benefit. Although participation in the NCDR registry is now voluntary, the lack of a mandate renders the data less valuable and subject to greater levels of bias on the basis of the centers that choose to continue participating. Alternative sources of data will be required to fill this gap.
Physicians must also take an active lead in improving the status quo. The current paradigm is easy to use. It takes a complex decision, such as the one to implant an ICD, and largely reduces it to a binary decision based on a single number: ejection fraction. While simplicity and ease of clinical application are laudable goals, effective ICD decision making is complex, requiring assessment of competing risks to develop patient‐specific predictions of potential benefit and harm, while simultaneously incorporating patient perspectives on what life with an ICD would mean for them. The 2017 American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines for management of ventricular arrhythmias and prevention of SCD give a class I recommendation to “shared decision making” such that “patients considering implantation of a new ICD or replacement of an existing ICD for low battery should be informed of their individual risk of SCD and nonsudden death from heart failure or noncardiac conditions and the effectiveness, safety and potential complications of the ICD in light of their health goals, preferences and values.”59 The recent CMS coverage update also included a requirement for a shared decision‐making interaction as a condition for coverage before primary prevention ICD implantation.55 It is essential that physicians and hospitals establish processes that allow for high‐quality shared decision making. Payors should support these efforts by reimbursing for time and effort spent in shared decision making regardless of whether the decision is made to ultimately implant an ICD.
Finally, under the current paradigm, many patients with ICDs never derive benefit from the device. Conversely, it is also well recognized that some patients who may benefit from ICDs do not undergo implantation, and ICDs may be underutilized in at‐risk populations.60, 61 Many potential reasons may contribute to therapeutic inertia and ICD underusage. Among them, one potential reason may be perceptions among physicians and patients of low magnitude of benefit and large numbers needed to treat with ICDs to save a life under current implantation guidelines. If so, then efforts to better understand competing risks of sudden and nonsudden death and improvements in the ability to identify those who are most, or least, likely to benefit from ICD implantation may improve usage.
Conclusions
Relatively little has changed over the past decade in the way that ICD recipients are selected. Although many patients have benefited from device implantation on the basis of the current paradigm, it is also likely that many have been subjected to device implantation with almost no reasonable expectation of benefit. As the field of primary prevention ICD therapy matures, it is essential that future efforts seek to better identify patients who are or are not likely to benefit from devices. Patient‐specific tools to predict overall mortality and proportional SCD risk provide the best and most cost‐effective opportunity for individualizing decisions regarding ICD implantation and ushering in an era of precision medicine that has thus far been lacking under the current paradigm.
Disclosures
Dr Kramer is supported by the Greenwall Faculty Scholars Program in Bioethics and is a consultant to Circulatory Systems Advisory Panel of the Food and Drug Administration. Dr Levy has developed the Seattle Heart Failure and Seattle Proportional Risk Models. The copyright is owned by the University of Washington CoMotion. He is a consultant to Medtronic (Heartware HVAD), Impulse Dynamics (Optimizer), Abbott (CardioMems—Clinical Endpoint Committee), EBR Systems (SOLVE CRT—Clinical Endpoint Committee), and GE Healthcare (ADMIRE ICD ‐ Steering Committee). Research support NHLBI R21—Risk stratification for ICD generator changes in the NCDR ICD Registry. Dr Merchant has no disclosures to report.
J Am Heart Assoc. 2020;9:e015139 DOI: 10.1161/JAHA.119.015139.
References
- 1. Al‐Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. 2018;138:e272–e391. [DOI] [PubMed] [Google Scholar]
- 2. Buxton AE, Lee KL, Hafley GE, Pires LA, Fisher JD, Gold MR, Josephson ME, Lehmann MH, Prystowsky EN, MUSTT Investigators . Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study. J Am Coll Cardiol. 2007;50:1150–1157. [DOI] [PubMed] [Google Scholar]
- 3. Tung R, Zimetbaum P, Josephson ME. A critical appraisal of implantable cardioverter‐defibrillator therapy for the prevention of sudden cardiac death. J Am Coll Cardiol. 2008;52:1111–1121. [DOI] [PubMed] [Google Scholar]
- 4. Reeder HT, Shen C, Buxton AE, Haneuse SJ, Kramer DB. Joint shock/death risk prediction model for patients considering implantable cardioverter‐defibrillators. Circ Cardiovasc Qual Outcomes. 2019;12:e005675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML; Multicenter Automatic Defibrillator Implantation Trial III . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 6. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp‐Channing N, Davidson‐Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH; Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT) Investigators . Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 7. Epstein AE, DiMarco JP, Ellenbogen KA, III Estes NA, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO; American College of Cardiology F, American Heart Association Task Force on Practice Guidelines and Heart Rhythm Society . 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device‐based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2013;127:e283–e352. [DOI] [PubMed] [Google Scholar]
- 8. Raphael C, Briscoe C, Davies J, Ian Whinnett Z, Manisty C, Sutton R, Mayet J, Francis DP. Limitations of the New York Heart Association functional classification system and self‐reported walking distances in chronic heart failure. Heart. 2007;93:476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kramer DB, Kennedy KF, Noseworthy PA, Buxton AE, Josephson ME, Normand SL, Spertus JA, Zimetbaum PJ, Reynolds MR, Mitchell SL. Characteristics and outcomes of patients receiving new and replacement implantable cardioverter‐defibrillators: results from the NCDR. Circ Cardiovasc Qual Outcomes. 2013;6:488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, Estes NA III, Greenberg H, Hall WJ, Huang DT, Kautzner J, Klein H, McNitt S, Olshansky B, Shoda M, Wilber D, Zareba W; MADIT‐RIT Investigators . Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367:2275–2283. [DOI] [PubMed] [Google Scholar]
- 11. Gasparini M, Proclemer A, Klersy C, Kloppe A, Lunati M, Ferrer JB, Hersi A, Gulaj M, Wijfels MC, Santi E, Manotta L, Arenal A. Effect of long‐detection interval vs standard‐detection interval for implantable cardioverter‐defibrillators on antitachycardia pacing and shock delivery: the ADVANCE III randomized clinical trial. JAMA. 2013;309:1903–1911. [DOI] [PubMed] [Google Scholar]
- 12. Tan VH, Wilton SB, Kuriachan V, Sumner GL, Exner DV. Impact of programming strategies aimed at reducing nonessential implantable cardioverter defibrillator therapies on mortality: a systematic review and meta‐analysis. Circ Arrhythm Electrophysiol. 2014;7:164–170. [DOI] [PubMed] [Google Scholar]
- 13. Kramer DB, Reynolds MR, Normand SL, Parzynski CS, Spertus JA, Mor V, Mitchell SL. Hospice use following implantable cardioverter‐defibrillator implantation in older patients: results from the national cardiovascular data registry. Circulation. 2016;133:2030–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Merchant FM, Jones P, Wehrenberg S, Lloyd MS, Saxon LA. Incidence of defibrillator shocks after elective generator exchange following uneventful first battery life. J Am Heart Assoc. 2014;3:001289 DOI: 10.1161/JAHA.114.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Merchant FM, Quest T, Leon AR, El‐Chami MF. Implantable cardioverter‐defibrillators at end of battery life: opportunities for risk (Re)‐stratification in ICD recipients. J Am Coll Cardiol. 2016;67:435–444. [DOI] [PubMed] [Google Scholar]
- 16. Merchant FM, Binney Z, Patel A, Li J, Peddareddy LP, El‐Chami MF, Leon AR, Quest T. Prevalence, predictors, and outcomes of advance directives in implantable cardioverter‐defibrillator recipients. Heart Rhythm. 2017;14:830–836. [DOI] [PubMed] [Google Scholar]
- 17. Poole JE, Gleva MJ, Mela T, Chung MK, Uslan DZ, Borge R, Gottipaty V, Shinn T, Dan D, Feldman LA, Seide H, Winston SA, Gallagher JJ, Langberg JJ, Mitchell K, Holcomb R, REPLACE Registry Investigators . Complication rates associated with pacemaker or implantable cardioverter‐defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation. 2010;122:1553–1561. [DOI] [PubMed] [Google Scholar]
- 18. Hsu JC, Varosy PD, Bao H, Dewland TA, Curtis JP, Marcus GM. Cardiac perforation from implantable cardioverter‐defibrillator lead placement: insights from the national cardiovascular data registry. Circ Cardiovasc Qual Outcomes. 2013;6:582–590. [DOI] [PubMed] [Google Scholar]
- 19. Sanders GD, Hlatky MA, Owens DK. Cost‐effectiveness of implantable cardioverter‐defibrillators. N Engl J Med. 2005;353:1471–1480. [DOI] [PubMed] [Google Scholar]
- 20. Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. [DOI] [PubMed] [Google Scholar]
- 21. Santangeli P, Epstein AE. Sudden cardiac death: lessons learned from cardiac implantable rhythm devices. Card Electrophysiol Clin. 2017;9:749–759. [DOI] [PubMed] [Google Scholar]
- 22. Deyell MW, Krahn AD, Goldberger JJ. Sudden cardiac death risk stratification. Circ Res. 2015;116:1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TD, Ismail NA, Dweck MR, Di Pietro E, Roughton M, Wage R, Daryani Y, O'Hanlon R, Sheppard MN, Alpendurada F, Lyon AR, Cook SA, Cowie MR, Assomull RG, Pennell DJ, Prasad SK. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896–908. [DOI] [PubMed] [Google Scholar]
- 24. Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long‐term (8‐year) benefit of the implantable cardioverter‐defibrillator. J Am Coll Cardiol. 2012;59:2075–2079. [DOI] [PubMed] [Google Scholar]
- 25. Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, Zareba W, McNitt S, Andrews ML, MADIT‐II Investigators . Risk stratification for primary implantation of a cardioverter‐defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51:288–296. [DOI] [PubMed] [Google Scholar]
- 26. Shen L, Jhund PS, Petrie MC, Claggett BL, Barlera S, Cleland JGF, Dargie HJ, Granger CB, Kjekshus J, Kober L, Latini R, Maggioni AP, Packer M, Pitt B, Solomon SD, Swedberg K, Tavazzi L, Wikstrand J, Zannad F, Zile MR, McMurray JJV. Declining risk of sudden death in heart failure. N Engl J Med. 2017;377:41–51. [DOI] [PubMed] [Google Scholar]
- 27. Levy WC. Should nonischemic CRT candidates receive CRT‐P or CRT‐D? J Am Coll Cardiol. 2017;69:1679–1682. [DOI] [PubMed] [Google Scholar]
- 28. Disertori M, Rigoni M, Pace N, Casolo G, Mase M, Gonzini L, Lucci D, Nollo G, Ravelli F. Myocardial fibrosis assessment by LGE is a powerful predictor of ventricular tachyarrhythmias in ischemic and nonischemic LV dysfunction: a meta‐analysis. JACC Cardiovasc Imaging. 2016;9:1046–1055. [DOI] [PubMed] [Google Scholar]
- 29. Noseworthy PA, Newton‐Cheh C. Genetic determinants of sudden cardiac death. Circulation. 2008;118:1854–1863. [DOI] [PubMed] [Google Scholar]
- 30. Tse G, Yan BP. Traditional and novel electrocardiographic conduction and repolarization markers of sudden cardiac death. Europace. 2017;19:712–721. [DOI] [PubMed] [Google Scholar]
- 31. Zaman S, Kovoor P. Sudden cardiac death early after myocardial infarction: pathogenesis, risk stratification, and primary prevention. Circulation. 2014;129:2426–2435. [DOI] [PubMed] [Google Scholar]
- 32. Zhang Y, Guallar E, Blasco‐Colmenares E, Dalal D, Butcher B, Norgard S, Tjong FV, Eldadah Z, Dickfeld T, Ellenbogen KA, Marine JE, Tomaselli GF, Cheng A. Clinical and serum‐based markers are associated with death within 1 year of de novo implant in primary prevention ICD recipients. Heart Rhythm. 2015;12:360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Levy WC, Lee KL, Hellkamp AS, Poole JE, Mozaffarian D, Linker DT, Maggioni AP, Anand I, Poole‐Wilson PA, Fishbein DP, Johnson G, Anderson J, Mark DB, Bardy GH. Maximizing survival benefit with primary prevention implantable cardioverter‐defibrillator therapy in a heart failure population. Circulation. 2009;120:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shadman R, Poole JE, Dardas TF, Mozaffarian D, Cleland JG, Swedberg K, Maggioni AP, Anand IS, Carson PE, Miller AB, Levy WC. A novel method to predict the proportional risk of sudden cardiac death in heart failure: Derivation of the Seattle Proportional Risk Model. Heart Rhythm. 2015;12:2069–2077. [DOI] [PubMed] [Google Scholar]
- 35. Hess PL, Al‐Khatib SM, Han JY, Edwards R, Bardy GH, Bigger JT, Buxton A, Cappato R, Dorian P, Hallstrom A, Kadish AH, Kudenchuk PJ, Lee KL, Mark DB, Moss AJ, Steinman R, Inoue LY, Sanders G. Survival benefit of the primary prevention implantable cardioverter‐defibrillator among older patients: does age matter? An analysis of pooled data from 5 clinical trials. Circ Cardiovasc Qual Outcomes. 2015;8:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Elming MB, Nielsen JC, Haarbo J, Videbaek L, Korup E, Signorovitch J, Olesen LL, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjaer H, Brandes A, Thogersen AM, Gustafsson F, Egstrup K, Videbaek R, Hassager C, Svendsen JH, Hofsten DE, Torp‐Pedersen C, Pehrson S, Kober L, Thune JJ. Age and outcomes of primary prevention implantable cardioverter‐defibrillators in patients with nonischemic systolic heart failure. Circulation. 2017;136:1772–1780. [DOI] [PubMed] [Google Scholar]
- 37. Levy WC, Li Y, Reed SD, Zile MR, Shadman R, Dardas T, Whellan DJ, Schulman KA, Ellis SJ, Neilson M, O'Connor CM, HFACTION Investigators . Does the implantable cardioverter‐defibrillator benefit vary with the estimated proportional risk of sudden death in heart failure patients? JACC Clin Electrophysiol. 2017;3:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Levy WC, Hellkamp AS, Mark DB, Poole JE, Shadman R, Dardas TF, Anderson J, Johnson G, Fishbein DP, Lee KL, Linker DT, Bardy GH. Improving the use of primary prevention implantable cardioverter‐defibrillators therapy with validated patient‐centric risk estimates. JACC Clin Electrophysiol. 2018;4:1089–1102. [DOI] [PubMed] [Google Scholar]
- 39. Bilchick KC, Stukenborg GJ, Kamath S, Cheng A. Prediction of mortality in clinical practice for Medicare patients undergoing defibrillator implantation for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2012;60:1647–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Koller MT, Schaer B, Wolbers M, Sticherling C, Bucher HC, Osswald S. Death without prior appropriate implantable cardioverter‐defibrillator therapy: a competing risk study. Circulation. 2008;117:1918–1926. [DOI] [PubMed] [Google Scholar]
- 41. van der Heijden AC, van Rees JB, Levy WC, van der Bom JG, Cannegieter SC, de Bie MK, van Erven L, Schalij MJ, Borleffs CJ. Application and comparison of the FADES, MADIT, and SHFM‐D risk models for risk stratification of prophylactic implantable cardioverter‐defibrillator treatment. Europace. 2017;19:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Rees JB, Borleffs CJ, van Welsenes GH, van der Velde ET, Bax JJ, van Erven L, Putter H, van der Bom JG, Schalij MJ. Clinical prediction model for death prior to appropriate therapy in primary prevention implantable cardioverter defibrillator patients with ischaemic heart disease: the FADES risk score. Heart. 2012;98:872–877. [DOI] [PubMed] [Google Scholar]
- 43. Bell SP, Orr NM, Dodson JA, Rich MW, Wenger NK, Blum K, Harold JG, Tinetti ME, Maurer MS, Forman DE. What to expect from the evolving field of geriatric cardiology. J Am Coll Cardiol. 2015;66:1286–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bennett M, Parkash R, Nery P, Senechal M, Mondesert B, Birnie D, Sterns LD, Rinne C, Exner D, Philippon F, Campbell D, Cox J, Dorian P, Essebag V, Krahn A, Manlucu J, Molin F, Slawnych M, Talajic M. Canadian Cardiovascular Society/Canadian Heart Rhythm Society 2016 implantable cardioverter‐defibrillator guidelines. Can J Cardiol. 2017;33:174–188. [DOI] [PubMed] [Google Scholar]
- 45. Kober L, Thune JJ, Nielsen JC, Haarbo J, Videbaek L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjaer H, Brandes A, Thogersen AM, Gustafsson F, Egstrup K, Videbaek R, Hassager C, Svendsen JH, Hofsten DE, Torp‐Pedersen C, Pehrson S; DANISH Investigators. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–1230. [DOI] [PubMed] [Google Scholar]
- 46. Kristensen SL, Levy WC, Shadman R, Nielsen JC, Haarbo J, Videbaek L, Bruun NE, Eiskjaer H, Wiggers H, Brandes A, Thogersen AM, Hassager C, Svendsen JH, Hofsten DE, Torp‐Pedersen C, Pehrson S, Signorovitch J, Kober L, Thune JJ. Risk models for prediction of implantable cardioverter‐defibrillator benefit: insights from the DANISH trial. JACC Heart Fail. 2019;7:717–724. [DOI] [PubMed] [Google Scholar]
- 47. Kramer DB, Kennedy KF, Spertus JA, Normand SL, Noseworthy PA, Buxton AE, Josephson ME, Zimetbaum PJ, Mitchell SL, Reynolds MR. Mortality risk following replacement implantable cardioverter‐defibrillator implantation at end of battery life: results from the NCDR. Heart Rhythm. 2014;11:216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Madhavan M, Waks JW, Friedman PA, Kramer DB, Buxton AE, Noseworthy PA, Mehta RA, Hodge DO, Higgins AY, Webster TL, Witt CM, Cha YM, Gersh BJ. Outcomes after implantable cardioverter‐defibrillator generator replacement for primary prevention of sudden cardiac death. Circ Arrhythm Electrophysiol. 2016;9:e003283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kini V, Soufi MK, Deo R, Epstein AE, Bala R, Riley M, Groeneveld PW, Shalaby A, Dixit S. Appropriateness of primary prevention implantable cardioverter‐defibrillators at the time of generator replacement: are indications still met? J Am Coll Cardiol. 2014;63:2388–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee DS, Krahn AD, Healey JS, Birnie D, Crystal E, Dorian P, Simpson CS, Khaykin Y, Cameron D, Janmohamed A, Yee R, Austin PC, Chen Z, Hardy J, Tu JV; Investigators of the Ontario ICD Database . Evaluation of early complications related to de novo cardioverter defibrillator implantation insights from the Ontario ICD database. J Am Coll Cardiol. 2010;55:774–782. [DOI] [PubMed] [Google Scholar]
- 51. Krahn AD, Lee DS, Birnie D, Healey JS, Crystal E, Dorian P, Simpson CS, Khaykin Y, Cameron D, Janmohamed A, Yee R, Austin PC, Chen Z, Hardy J, Tu JV; Ontario ICD Database Investigators . Predictors of short‐term complications after implantable cardioverter‐defibrillator replacement: results from the Ontario ICD Database. Circ Arrhythm Electrophysiol. 2011;4:136–142. [DOI] [PubMed] [Google Scholar]
- 52. Agarwala V, Khozin S, Singal G, O'Connell C, Kuk D, Li G, Gossai A, Miller V, Abernethy AP. Real‐world evidence in support of precision medicine: clinico‐genomic cancer data as a case study. Health Aff (Millwood). 2018;37:765–772. [DOI] [PubMed] [Google Scholar]
- 53. James S, Rao SV, Granger CB. Registry‐based randomized clinical trials–a new clinical trial paradigm. Nat Rev Cardiol. 2015;12:312–316. [DOI] [PubMed] [Google Scholar]
- 54. Solomon SD, Pfeffer MA. The future of clinical trials in cardiovascular medicine. Circulation. 2016;133:2662–2670. [DOI] [PubMed] [Google Scholar]
- 55. Jensen TS, Chin J, Ashby L, Dolan D, Canos D, Hutter J. Decision Memo for Implantable Cardioverter Defibrillators (CAG‐00157R4). Centers for Medicare & Medicaid Services. 2018. Available at: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=288. Accessed March 17, 2019.
- 56. Ambrosy AP, Parzynski CS, Friedman DJ, Fudim M, Hernandez AF, Fonarow GC, Masoudi FA, Al‐Khatib SM. Is Time from last hospitalization for heart failure to placement of a primary prevention implantable cardioverter‐defibrillator associated with patient outcomes? Circulation. 2018;138:2787–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Betz JK, Katz DF, Peterson PN, Borne RT, Al‐Khatib SM, Wang Y, Hansen CM, McManus DD, Mathew JS, Masoudi FA. Outcomes among older patients receiving implantable cardioverter‐defibrillators for secondary prevention: from the NCDR ICD registry. J Am Coll Cardiol. 2017;69:265–274. [DOI] [PubMed] [Google Scholar]
- 58. Akar JG, Bao H, Jones P, Wang Y, Chaudhry SI, Varosy P, Masoudi FA, Stein K, Saxon LA, Curtis JP. Use of remote monitoring of newly implanted cardioverter‐defibrillators: insights from the patient related determinants of ICD remote monitoring (PREDICT RM) study. Circulation. 2013;128:2372–2383. [DOI] [PubMed] [Google Scholar]
- 59. Al‐Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72:1677–1749. [DOI] [PubMed] [Google Scholar]
- 60. Narayanan K, Reinier K, Uy‐Evanado A, Teodorescu C, Chugh H, Marijon E, Gunson K, Jui J, Chugh SS. Frequency and determinants of implantable cardioverter defibrillator deployment among primary prevention candidates with subsequent sudden cardiac arrest in the community. Circulation. 2013;128:1733–1738. [DOI] [PubMed] [Google Scholar]
- 61. Zhang L, Narayanan K, Chugh H, Shiota T, Zheng ZJ, Chugh SS. Factors influencing utilization of the primary prevention implantable defibrillator. PLoS One. 2015;10:e0121515. [DOI] [PMC free article] [PubMed] [Google Scholar]


