Abstract
Background
Pulmonary arterial hypertension (PAH) is a lethal disease. In resource‐limited countries PAH outcomes are worse because therapy costs are prohibitive. To improve global outcomes, noninvasive and widely available biomarkers that identify high‐risk patients should be defined. Serum chloride is widely available and predicts mortality in left heart failure, but its prognostic utility in PAH requires further investigation.
Methods and Results
In this study 475 consecutive PAH patients evaluated at the University of Minnesota and Vanderbilt University PAH clinics were examined. Clinical characteristics were compared by tertiles of serum chloride. Both the Kaplan‐Meier method and Cox regression analysis were used to assess survival and predictors of mortality, respectively. Categorical net reclassification improvement and relative integrated discrimination improvement compared prediction models. PAH patients in the lowest serum chloride tertile (≤101 mmol/L: hypochloremia) had the lowest 6‐minute walk distance and highest right atrial pressure despite exhibiting no differences in pulmonary vascular disease severity. The 1‐, 3‐, and 5‐year survival was reduced in hypochloremic patients when compared with the middle‐ and highest‐tertile patients (86%/64%/44%, 95%/78%/59%, and, 91%/79%/66%). After adjustment for age, sex, diuretic use, serum sodium, bicarbonate, and creatinine, the hypochloremic patients had increased mortality when compared with the middle‐tertile and highest‐tertile patients. The Minnesota noninvasive model (functional class, 6‐minute walk distance, and hypochloremia) was as effective as the French noninvasive model (functional class, 6‐minute walk distance, and elevated brain natriuretic peptide or N‐terminal pro–brain natriuretic peptide) for predicting mortality.
Conclusions
Hypochloremia (≤101 mmol/L) identifies high‐risk PAH patients independent of serum sodium, renal function, and diuretic use.
Keywords: biomarkers, chloride, pulmonary arterial hypertension, right atrial pressure, right ventricular failure
Subject Categories: Quality and Outcomes, Mortality/Survival, Pulmonary Hypertension
Clinical Perspective
What Is New?
Our data show that low serum chloride levels are associated with right ventricular dysfunction and increased mortality in pulmonary arterial hypertension independent of serum sodium levels.
A combination of serum chloride, functional class, and 6‐minute walk distance predicts mortality in patients with pulmonary arterial hypertension similar to the French noninvasive prediction model with age, functional class, 6‐minute walk distance, and serum N‐terminal pro–brain natriuretic peptide levels.
What Are the Clinical Implications?
Serum chloride is an inexpensive and noninvasive way to identify high‐risk patients with pulmonary arterial hypertension who may require more intensive therapy and monitoring.
A combination of serum chloride, functional class, and 6‐minute walk distance can be helpful for mortality prediction in patients with pulmonary arterial hypertension.
Introduction
Pulmonary arterial hypertension (PAH) is a rare and lethal disease characterized by obstructive vascular remodeling and vasoconstriction of the precapillary arterioles and ultimately causes right ventricular dysfunction and death.1, 2 Despite progress in understanding the pathogenesis and treatment of PAH in the past 2 decades, it remains a fatal illness with a median survival of 5 to 7 years.1 PAH is frequently encountered in resource‐limited countries that often have limited access to tools that are employed to risk stratify patients with pulmonary hypertension (PH) to help guide diagnosis and inform decisions on therapy implementation.3 Risk assessment is pivotal in the management of PAH to improve outcomes, and several prognostic markers have been recommended by the current guidelines for risk stratification and treatment selection in patients with PAH.4 However, many of these prognostic parameters require advanced or invasive testing.4 Thus, there is an unmet need to define inexpensive and widely available biomarkers for prognostication and identification of patients at greatest need for therapy.
There is precedence for noninvasive markers having utility in risk stratification in PAH. For example, hyponatremia is associated with right ventricular dysfunction and reduced survival in PAH.5 However, recent data from the heart failure literature suggest hypochloremia, and not hyponatremia, is the more predictive electrolyte abnormality for predicting poor outcomes in acute decompensated left heart failure6 and in chronic heart failure.7, 8 It is unknown whether serum chloride could serve as a simple, inexpensive, and widely available parameter for prognostication in PAH. There are limited data on the prognostic value of serum chloride levels in PAH patients. In idiopathic or heritable PAH patients low serum chloride levels (≤100 mmol/L), measured 6 months after diagnosis, were associated with higher mortality rates, even after adjustment for age, sex, pulmonary vascular resistance, and use of diuretics or prostacyclin.9 Multicenter studies evaluating the utility of serum chloride levels to identify high‐risk PAH are lacking.
Thus, the aim of this article is to assess the prognostic value of serum chloride levels at the time of diagnosis in PAH patients referred to 2 designated PH centers. We hypothesized that a low serum chloride level is a noninvasive predictor of mortality in patients with PAH, independent of serum sodium, renal function, and diuretic use. We further tested how a noninvasive mortality prediction model using serum chloride compared with the French noninvasive prediction model.
Methods
Data Availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Population
The University of Minnesota and Vanderbilt University Medical Center (VUMC) Institutional Review Boards approved this study. We studied adult PAH patients enrolled in the Minnesota Pulmonary Hypertension Repository10 and VUMC PH patient database.11
The Minnesota Pulmonary Hypertension Repository is a customized patient database that collects specific variables on every patient treated at the University of Minnesota Pulmonary Hypertension Clinic (March 2014 to present).10 Data are collected by chart review and entered using an internet‐based electronic data‐capture system. We collected baseline demographics, clinical characteristics including comorbidities and medications, laboratory values, echocardiographic variables, and hemodynamic variables at the time of diagnosis. Patients diagnosed before March 2014 were entered retrospectively. All patients gave informed consent for participation in the repository.
Patients in the VUMC PH database were identified using the Vanderbilt Synthetic Derivative, a mirror‐image, deidentified version of the electronic medical record containing data on ≈2.5 million patients. 11 Clinical and hemodynamic data from all patients undergoing right heart catheterization at VUMC between 1998 and 2014 were extracted as described. In brief, we collected laboratory data, clinical diagnoses, and echocardiographic data closest in time to, but not >6 months before or after, the date of right heart catheterization. Only medications prescribed before right heart catheterization were extracted. Comorbidities were defined by International Classification of Diseases, Ninth Revision (ICD‐9) codes in the medical record before right heart catheterization or as defined by previously validated algorithms.12
We identified adult patients ≥18 years of age with PAH in the Minnesota PH registry and the VUMC PH database. The diagnosis of PAH required the following updated PAH definition: mean pulmonary arterial pressure (mPAP) ≥20 mm Hg, pulmonary vascular resistance (PVR) ≥3.0 Wood units, and pulmonary capillary wedge pressure ≤15 mm Hg.13 Other etiologies of pulmonary hypertension were ruled out using pulmonary function tests, echocardiograms, computerized tomography scans of lungs, ventilation‐perfusion scans, and clinical assessments. Patients were excluded if they had obstructive lung disease diagnosed by reduced expiratory flow rates (forced expiratory volume in 1 second/forced vital capacity <75% predicted); more than mild interstitial lung disease diagnosed by reduced total lung capacity <60%; chronic pulmonary thromboembolic disease diagnosed by ventilation/perfusion scan (high or intermediate probability), contrast‐enhanced chest computerized tomography, or pulmonary angiography. Patients meeting hemodynamic criteria for PAH at VUMC underwent manual chart review to confirm the diagnosis.
A total of 513 PAH patients in the University of Minnesota PH registry (n=180) and the VUMC PH database (n=333) met the study inclusion and exclusion criteria. Of these 513 patients, we excluded 38 patients who did not have a baseline serum chloride level. The remaining 475 patients formed our study cohort.
Serum Chloride Levels
We collected serum chloride levels at the time of initial referral to our center. Serum chloride levels were measured from the peripheral venous blood samples drawn during routine clinic visits or diagnostic right heart catheterization. The mean duration between collection of serum chloride sample collection and the diagnostic right heart catheterization at the time of referral was 5 (25% to 75%: 0‐99) days.
Outcomes
The primary outcome was time to all‐cause mortality. All patients were followed regularly at the University of Minnesota and VUMC PH clinic every 3 to 6 months. For the Minnesota PH registry, vital statistics were obtained for all patients by chart review and Minnesota Death index. For the VUMC PH database, the Synthetic Derivative is linked to the Social Security death index to ascertain vital status.
Covariates
We analyzed the following baseline covariates available in the University of Minnesota PH repository and VUMC PH database: age, sex, body mass index, concomitant medications, 6‐minute walk distance (6MWD), laboratory data including serum sodium, serum bicarbonate, serum creatinine, serum NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) or brain natriuretic peptide (BNP) levels, echocardiographic data, and invasive hemodynamic data. Echocardiographic analysis was performed as described to semiquantitatively examine right ventricular size and function.14 The following hemodynamic variables were analyzed: right atrial pressure, mean pulmonary artery pressure, pulmonary capillary wedge pressure, cardiac output, cardiac index, pulmonary artery hemoglobin saturation, PVR, and pulmonary artery compliance. PVR was calculated in Wood units as the difference between mPAP and pulmonary capillary wedge pressure divided by the cardiac output. Stroke volume (mL) was calculated from cardiac output and heart rate. Pulmonary arterial compliance (mL/mm Hg) was calculated as the ratio of stroke volume to the pulmonary artery pulse pressure, as previously described.15
Statistical Analyses
Categorical data are expressed as frequency and proportions, and continuous data are presented as mean±standard deviation or as median with interquartile range. Baseline characteristics of patients in different tertiles of serum chloride were compared using 1‐way ANOVA for normally distributed continuous variables and Kruskal‐Wallis tests for nonnormally distributed continuous variables. A chi square or Fisher exact test was used to compare proportions of normally distributed and nonnormally distributed categorical variables, respectively. To understand the clinical correlates of serum chloride level in PAH patients, we performed univariate linear regression analysis with serum chloride level as the dependent variable. To assess the impact of serum chloride on mortality, we used Kaplan‐Meier survival analysis. The date of diagnostic right heart catheterization was considered as the date of entry into the study. The primary end‐point was all‐cause mortality. Log rank test was used to compare survival between tertiles of serum chloride. We used multivariable Cox's proportional hazards analyses to assess the effect of serum chloride on mortality. We adjusted for predefined covariates that are known to be associated with hypochloremia including age, sex, diuretic use, serum sodium, serum bicarbonate, and serum creatinine in a stepwise forward and backward fashion to determine the independent impact of serum chloride on survival. The proportional hazards assumption was tested in all models. Finally, to examine the predictive value of hypochloremia (<101 mmol/L), we constructed 2 models for predicting mortality. The first model was comprised of functional class, 6MWD, and presence of hypochloremia (Minnesota Model). The second model was based on the French noninvasive prediction model of functional class, 6MWD, and presence of elevated brain natriuretic peptide (BNP) or N‐terminal pro‐BNP.16 We imputed data for model comparisons to ensure a uniform number of patients had data on 6MWT, BNP/N‐terminal proBNP, World Health Organization Functional Class, and chloride levels. We then quantified model performance using chi‐squared likelihood ratios, Akaike information criteria, Bayesian information criterion, the category‐free (continuous) net reclassification improvement, and integrated discrimination improvement.17, 18 All statistical analyses were performed using Stata software Version 15 (Stata Corp LP, College Station, TX). A P‐value of <0.05 was considered statistically significant.
Results
Baseline Characteristics
Our PAH cohort comprised 475 patients with 304 patients coming from the VUMC PH registry and 171 patients coming from the University of Minnesota PH Registry. There was a female predominance (75%), and the mean age was 52±15 years. Of the 475 patients, 298 patients (63%) were PAH‐specific vasodilator treatment naive at the time of referral to our centers. Patients exhibited functional impairments with a mean 6MWD of 332±117 m. Mean serum chloride level was 104±5 mmol/L. Figure 1 illustrates the distribution of serum chloride levels in our study cohort. The invasive hemodynamic characterization demonstrated severe PAH in our cohort (mean pulmonary artery pressure [mPAP] 48±13 mm Hg, mean pulmonary vascular resistance [PVR] 10.3±5.5 Wood units, mean cardiac output 4.4±1.5 L/min, and mean pulmonary arterial compliance 1.4±0.8 mL/mm Hg). Most patients had both right ventricular (RV) dilation (81%) and RV dysfunction (71%) on echocardiographic analysis (Table 1).
Figure 1.
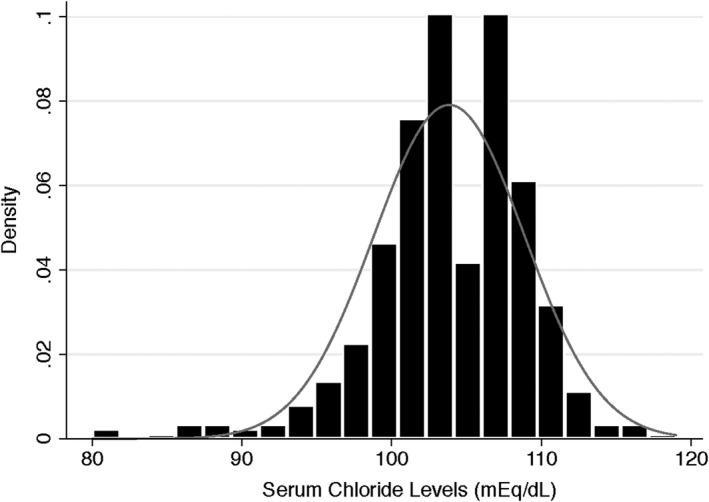
Distribution of serum chloride levels in the study cohort.
Table 1.
Clinical Characteristics of Study Cohort by Tertiles of Serum Chloride Levels
| Characteristics | Total Cohort (n=475) | Lowest Tertile (n=120) | Middle Tertile (n=167) | Highest Tertile (n=188) | P Value |
|---|---|---|---|---|---|
| Age, y | 52±15 | 54±14 | 53±14 | 50±15 | 0.02 |
| Female, n (%) | 357 (75) | 96 (80) | 124 (74) | 137 (73) | 0.35 |
| Body mass index, kg/m2 | 28.7±6.9 | 28.7±7.0 | 28.9±7.3 | 28.6±6.6 | 0.92 |
| Medications, n (%) | |||||
| Diuretics | 265 (56) | 86 (72) | 102 (61) | 77 (41) | <0.001 |
| Calcium channel blockers | 84 (18) | 21 (18) | 30 (18) | 33 (18) | 0.99 |
| Phosphodiesterase‐5 inhibitors | 94 (20) | 23 (19) | 30 (18) | 41 (22) | 0.67 |
| Endothelin receptor antagonists | 71 (15) | 22 (18) | 22 (13) | 27 (14) | 0.46 |
| Prostacyclins | 80 (17) | 25 (21) | 28 (17) | 27 (14) | 0.34 |
| PAH‐specific treatment naive | 298 (63) | 66 (55) | 109 (65) | 123 (65) | 0.128 |
| Etiology of PAH, n (%) | 0.04 | ||||
| Idiopathic | 169 (35) | 55 (46) | 53 (32) | 61 (32) | |
| Heritable | 20 (4) | 1 (1) | 6 (3) | 13 (7) | |
| Drug‐induced (anorexigen or methamphetamine) | 15 (3) | 3 (3) | 5 (3) | 7 (4) | |
| Connective tissue disease | 151 (32) | 42 (35) | 55 (33) | 54 (28) | |
| Congenital heart disease | 51 (11) | 4 (3) | 25 (15) | 22 (12) | |
| Human immunodeficiency virus infection | 10 (2) | 2 (2) | 3 (2) | 5 (3) | |
| Portopulmonary hypertension | 56 (12) | 12 (10) | 20 (12) | 24 (13) | |
| Others | 3 (1) | 1 (1) | 0 (0) | 2 (1) | |
| 6‐minute walk test | |||||
| Distance, m (n=358) | 332±117 | 305±130 (n=84) | 339±110 (n=130) | 344±113 (n=144) | 0.04 |
| Laboratory values | |||||
| Serum sodium, mmol/L (n=473) | 138±4 | 136±5 | 139±3 | 140±3 | <0.001 |
| Serum chloride, mmol/L (n=475) | 104±5 | 97±4 | 103±1 | 108±2 | <0.001 |
| Serum bicarbonate, mmol/L (n=473) | 25±4 | 28±5 | 26±3 | 24±3 | <0.001 |
| Serum creatinine, mg/dL (n=475) | 1.1±0.8 | 1.2±1.0 | 1.0±0.9 | 1.0±0.3 | 0.06 |
| Serum BNP, pg/dL (n=240)a | 513±752 | 835±1208 | 401±411 | 404±513 | 0.11 |
| Serum NT‐proBNP, pg/dL (n=152)b | 3085±6371 | 5752±11 433 | 2692±3368 | 2137±4224 | 0.13 |
| Serum hemoglobin, g/dL (n=472) | 13.6±2.5 | 13.0±2.0 | 14.2±2.4 | 13.5±2.3 | <0.001 |
| Echocardiography | |||||
| Left ventricular EF, % (n=432) | 62±13 | 62±13 | 62±13 | 62±13 | 0.83 |
| Right ventricular enlargement (n=447) | 362 (81) | 98 (84) | 125 (80) | 139 (80) | 0.66 |
| Right ventricular dysfunction (n=443) | 316 (71) | 89 (77) | 113 (72) | 114 (67) | 0.20 |
| Hemodynamics | |||||
| Heart rate, beats/min (n=371) | 78±14 | 82±13 | 79±13 | 76±14 | 0.005 |
| Mean right atrial, mm Hg (n=454) | 8±5 | 10±6 | 8±6 | 8±5 | <0.001 |
| Mean PAP, mm Hg (n=459) | 48±13 | 47±12 | 50±15 | 48±12 | 0.35 |
| PCWP, mm Hg (n=475) | 9±4 | 9±4 | 9±4 | 9±3 | 0.24 |
| Cardiac output, L/min (n=442) | 4.4±1.5 | 4.4±1.6 | 4.5±1.5 | 4.3±1.4 | 0.51 |
| Cardiac index, L/min per m2 (n=394) | 2.4±0.7 | 2.4±0.8 | 2.5±0.8 | 2.3±0.7 | 0.15 |
| Pulmonary arterial saturation (n=406) | 64±9 | 61±9 | 65±9 | 64±9 | 0.006 |
| PVR, WU (n=427) | 10.3±5.5 | 10.0±5.7 | 10.6±6.1 | 10.3±5.0 | 0.50 |
| PAC, mL/mm Hg (n=332) | 1.4±0.8 | 1.3±0.7 | 1.4±0.9 | 1.4±0.7 | 0.94 |
BNP indicates brain natriuretic peptide; EF, ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PAC, pulmonary arterial compliance; PAH, pulmonary arterial hypertension; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance.
Vanderbilt University Medical Center cohort only.
University of Minnesota cohort only.
Comparison of Baseline Characteristics by Tertiles of Serum Chloride
We divided our cohort into tertiles based on serum chloride levels (≤101, 102‐105, and >105 mmol/L) and examined patient characteristics. Hypochloremic patients, defined as those in the lowest tertile, were older and more likely to be treated with a diuretic, but there were no differences in PAH‐specific therapy at the time of referral (Table 1). Hypochloremic patients were more likely to have idiopathic or connective tissue–associated PAH and were less likely to have congenital heart disease. Hypochloremic patients had the shortest 6MWD. On laboratory examination, hypochloremic patients had the lowest sodium and highest bicarbonate values with a trend toward higher creatinine (Table 1). There were no differences in the proportion of patients with RV enlargement and dysfunction across the tertiles of serum chloride levels. Finally, on hemodynamic evaluation, hypochloremic patients had a higher heart rate, right atrial pressure, and lower pulmonary arterial hemoglobin saturation despite having no differences in mPAP, PVR, or pulmonary arterial compliance (Table 1).
Clinical Correlates of Serum Chloride
To understand what serum chloride levels reflected biologically, we performed a systematic univariate regression analysis between serum chloride and all available clinical covariates. As expected, lower serum chloride levels were associated with lower serum sodium levels (Figure S1) and higher serum bicarbonate levels (Figure S1), diuretic use, and higher serum creatinine levels (Table 2). However, lower serum chloride levels were also associated with measures of RV impairment including higher BNP levels, the presence of RV dysfunction on echocardiogram, and higher right atrial pressure (Table 2). Finally, lower serum chloride levels were associated with lower 6MWD (Table 2). In summary, these data demonstrate that hypochloremia is associated with RV dysfunction and reduced exercise capacity.
Table 2.
Clinical Correlates of Serum Chloride in Pulmonary Artery Hypertension
| Variable | β‐Coefficient | 95% CI | P Value |
|---|---|---|---|
| Age, y | −0.038 | −0.07, −0.007 | 0.014 |
| Female sex | −0.20 | −1.25, 0.85 | 0.71 |
| Body mass index, kg/m2 | 0.025 | −0.042, 0.093 | 0.47 |
| Use of diuretics | −2.37 | −3.26, −1.48 | <0.001 |
| PDE‐5 inhibitor | 0.61 | −0.53, 1.75 | 0.29 |
| ERA | −0.37 | −1.65, 0.90 | 0.56 |
| 6MWD | 0.005 | 0.0006, 0.009 | 0.03 |
| Sodium, mmol/L | 0.78 | 0.67, 0.88 | <0.001 |
| Bicarbonate, mmol/L | −0.75 | −0.84, −0.65 | <0.001 |
| Creatinine, mg/dL | −0.76 | −1.35, −0.16 | 0.013 |
| BNP, pg/dLa | −0.002 | −0.003, −0.0008 | <0.001 |
| NT‐proBNP, pg/dLb | −0.00009 | −0.0002, 0.00002 | 0.11 |
| LVEF, % | 0.018 | −0.018, 0.054 | 0.317 |
| RV enlargement | −0.40 | −1.59, 0.80 | 0.517 |
| RV dysfunction | −1.53 | −2.57, −0.49 | 0.004 |
| Heart rate, beats per min | −0.047 | −0.086, −0.009 | 0.015 |
| RA pressure, mm Hg | −0.13 | −0.22, −0.047 | 0.003 |
| mPAP, mm Hg | 0.014 | −0.021, 0.049 | 0.429 |
| PCWP, mm Hg | −0.020 | −0.144, 0.105 | 0.755 |
| Cardiac output, L/min | −0.177 | −0.493, 0.139 | 0.272 |
| Cardiac index, L/min per m2 | −0.445 | −1.13, 0.238 | 0.201 |
| PA saturation, % | 0.057 | 0.005, 0.109 | 0.031 |
| PVR, Wood Units | 0.021 | −0.067, 0.108 | 0.643 |
| PAC, mL/mm Hg | −0.103 | −0.812, 0.605 | 0.775 |
6MWD indicates 6‐minute walk distance; BNP, brain natriuretic peptide; ERA, endothelin receptor antagonists; LVEF, left ventricular ejection fraction; mPAP, mean pulmonary artery pressure; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PA, pulmonary artery saturation; PAC, pulmonary arterial compliance; PCWP, pulmonary capillary wedge pressure; PDE‐5 inhibitors, phosphodiesterase‐5 inhibitors; PVR, pulmonary vascular resistance; RA, right atrial; RV, right ventricle.
Vanderbilt University Medical Center cohort only.
University of Minnesota cohort only.
Hypochloremia Is a Predictor of Mortality in PAH
During a median follow‐up of 4.6 years, there were 217 deaths. The 1‐, 3‐, and 5‐year survival rates in the lowest to highest tertiles were 86%/64%/44%, 95%/78%/59%, and, 91%/79%/66%, respectively (log rank test P=0.004) (Figure 2). On unadjusted Cox proportional hazards analysis, hypochloremia was associated with a 65% increased hazard of mortality when compared with the middle tertile of serum chloride levels and an 83% increased hazard of mortality when compared with the highest tertile of serum chloride (Table 3). With serum chloride levels as a continuous variable, every 1 mmol/L decrease in serum chloride level was associated with a 4% increase in the hazard of mortality (hazard ratio 1.04 [95% CI 1.02‐1.06], P=0.003). On multivariate analysis, after adjustment for age, sex, diuretic use, serum sodium levels, serum bicarbonate levels, and serum creatinine levels, hypochloremia was associated with 58% increased hazards of mortality compared with the middle tertile of serum chloride levels and 65% increased hazard of mortality compared with the highest tertile of serum chloride levels (Table 3). Serum chloride level, when used as a continuous variable, was significantly and inversely associated with increased mortality after adjustment for age, sex, diuretic use, serum sodium levels, serum bicarbonate levels, and serum creatinine levels in the multivariable analysis (hazard ratio 1.05 [95% CI 1.02‐1.08], P=0.004) only in the backward selection model. When we separated our cohort based on sex, both males and females with low serum chloride had increased mortality (Figure S2).
Figure 2.
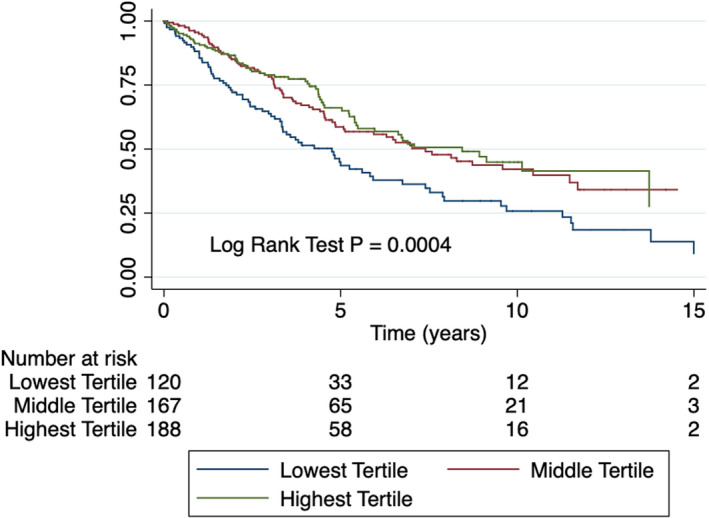
Kaplan‐Meier curves depicting survival stratified by tertiles of serum chloride. Patients in the lowest chloride tertile have reduced survival compared with the middle and highest tertiles.
Table 3.
Unadjusted and Adjusted Cox Proportional Hazards Ratio for the Lowest Tertile of Serum Chloride Levels as a Predictor of Mortality in PAH
| Reference Group | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Total cohort (N=475) | ||
| Unadjusted | ||
| Middle tertile of serum chloride | 1.65 (1.20‐2.27) | 0.002 |
| Highest tertile of serum chloride | 1.83 (1.31‐2.55) | <0.001 |
| Adjusteda | ||
| Middle tertile of serum chloride | 1.58 (1.14‐2.17) | 0.005 |
| Highest tertile of serum chloride | 1.65 (1.21‐2.25) | 0.004 |
| After excluding patients on diuretics (n=210) | ||
| Unadjusted | ||
| Middle tertile of serum chloride | 2.46 (1.39‐4.35) | 0.002 |
| Highest tertile of serum chloride | 2.64 (1.56‐4.48) | <0.001 |
| Adjusteda | ||
| Middle tertile of serum chloride | 1.38 (0.67‐2.83) | 0.379 |
| Highest tertile of serum chloride | 2.18 (1.34‐3.54) | 0.002 |
| After excluding patients on PAH‐specific vasodilator therapy at the time of referral (n=298) | ||
| Unadjusted | ||
| Middle tertile of serum chloride | 1.78 (1.17‐2.70) | 0.007 |
| Highest tertile of serum chloride | 1.85 (1.21‐2.81) | 0.004 |
| Adjusteda | ||
| Middle tertile of serum chloride | 1.46 (0.86‐2.48) | 0.159 |
| Highest tertile of serum chloride | 1.87 (1.27‐2.75) | <0.001 |
PAH indicates pulmonary artery hypertension.
Adjusted for age, sex, diuretic use, serum sodium, serum bicarbonate, and serum creatinine.
Hypochloremia Predicts Mortality in the Absence of Diuretic Usage
Although we adjusted for diuretic use in our model, in an attempt to completely avoid the confounding use of diuretics on serum chloride levels, we also examined the prognostic ability of hypochloremia in PAH patients not on diuretics. There were 210 patients who were not on diuretics at the time of referral. In this group, on unadjusted hazards analysis, hypochloremia was associated with a 2.46‐fold increased risk of mortality compared with the middle‐tertile patients and a 2.64‐fold increased risk of mortality compared with the highest‐tertile patients (Table 3 and Figure 3). After multivariate adjustment, the difference in mortality was no longer statistically significant between the hypochloremic and the middle‐tertile group, but hypochloremic patients had a 2.18‐fold increased risk of mortality when compared with the highest‐tertile patients (Table 3 and Figure S3).
Figure 3.
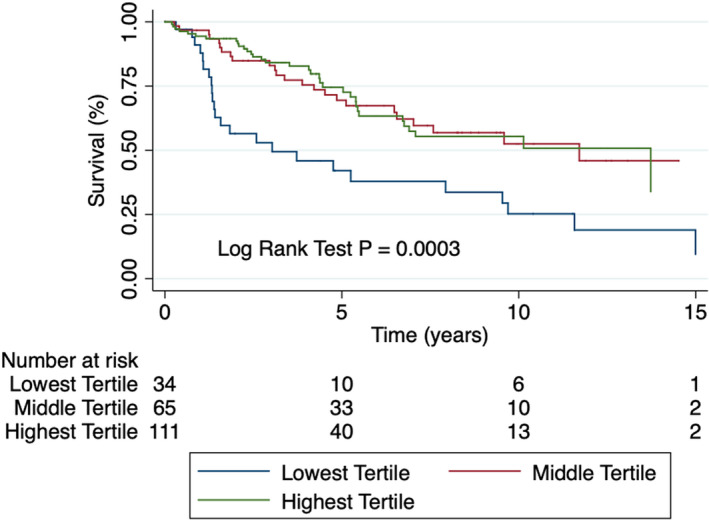
Kaplan‐Meier curve depicting survival stratified by tertiles of serum chloride in PAH patients not on diuretics. Hypochloremic patients have reduced survival compared with the highest‐ tertile patients after multivariate correction. PAH indicates pulmonary arterial hypertension.
Hypochloremia Predicts Mortality in PAH‐Specific Treatment–Naive Patients
When we restricted our analysis to PAH‐specific treatment–naïve patients at the time of referral, we obtained similar results. On unadjusted analysis, hypochloremia was associated with a 1.87‐fold increased risk of mortality compared with the middle tertile of patients and a 1.85‐fold increased risk of mortality compared with the highest tertile patients (Figure 4, Figure S3, and Table 3). After adjusting for age, sex, diuretic use, serum sodium levels, serum bicarbonate levels, and serum creatinine, the difference in mortality was no longer statistically significant between the hypochloremic and the middle tertile group, but hypochloremic patients had a 1.87‐fold increased risk of mortality when compared with the highest tertile group (Table 3).
Figure 4.
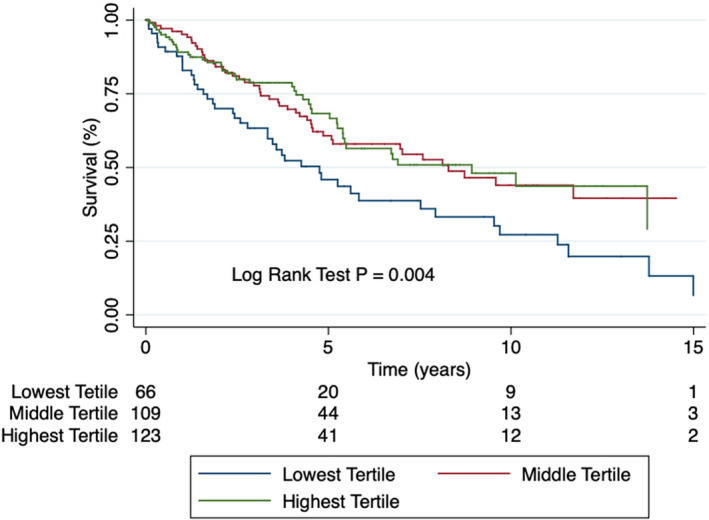
Kaplan‐Meier curve depicting survival stratified by tertiles of serum chloride in PAH‐specific treatment–naïve patients. Hypochloremic patients have reduced survival as compared with the highest tertile patients. PAH indicates pulmonary arterial hypertension.
Hypochloremia in a Noninvasive Model for Predicting Mortality
Finally, we examined the utility of hypochloremia for predicting mortality in a noninvasive model. We compared the French noninvasive prediction model16 to our model, which included 3 readily available, noninvasive variables: functional class, 6MWD, and presence of hypochloremia. Both models have similar predictive values in this multicenter cohort as assessed by chi‐squared likelihood ratios, Akaike information criteria, Bayesian information criterion, category‐free (continuous) net reclassification improvement, and integrated discrimination improvement (Tables 4 and 5).
Table 4.
Characterization of French and Minnesota Noninvasive Prediction Models for Predicting Mortality
| Model | Chi‐Squared Likelihood Ratio | Akaike Information Criterion | Bayesian Information Criterion | Harrell C Statistic |
|---|---|---|---|---|
| French | 55.0 | 2038.9 | 2321.4 | 0.64 |
| Minnesota | 55.3 | 2038.6 | 2321.0 | 0.64 |
Table 5.
Comparison of French and Minnesota Models for Predicting Mortality
| Baseline Model | New Model | Summary of Continuous Net Reclassification Index (95% CI) | Continuous Event and Nonevent Net Reclassification Indices (95% CI) | Summary of Integrative Discrimination Improvement (95% CI) | Event and Nonevent Integrative Discrimination Improvement (95% CI) |
|---|---|---|---|---|---|
| French | Minnesota | 0.342 (0.177, 0.501) | Event −0.309 (−0.427, −0.181) Nonevent 0.651 (0.547, 0.740) | 0.025 (0.002, 0.062) |
Event 0.013 (0.001, 0.033) Nonevent 0.01 (0.001, 0.028) |
Discussion
In this article we show that serum chloride levels are associated with measures of right ventricular dysfunction and impaired exercise capacity despite having no significant relationship with pulmonary vascular disease severity as assessed by mPAP, PVR, and pulmonary arterial compliance. Moreover, hypochloremic PAH patients have evidence of right heart failure and functional impairment with higher right atrial pressure and reduced 6MWD. Importantly, hypochloremia is associated with increased mortality even after correction for age, sex, serum sodium, serum bicarbonate, serum creatinine, and use of diuretics. Furthermore, in patients not on diuretics and those who are PAH‐specific treatment naive, hypochloremia remains a risk factor for mortality. Finally, we show that a new Minnesota noninvasive model (functional class, 6MWD, and hypochloremia) has a nearly identical prediction value to that of the French noninvasive model.16 Collectively, these results validate serum chloride as a widely available and inexpensive biomarker for prognostication in PAH patients.
Recently Naal et al showed that serum chloride levels below 100 mmol/L at 6 months postdiagnosis are associated with increased risk of mortality in patients with idiopathic or heritable PAH.9 Our results are slightly different from the results of Naal et al. Although both groups find that hypochloremia predicts survival, we show that hypochloremia is associated with worse right ventricular function because hypochloremic patients have higher right atrial pressure (Table 2). In contrast, Naal et al did not observe evidence of RV dysfunction in their hypochloremic group.9 Moreover, we show that the baseline serum chloride level at the time of diagnosis is an independent predictor of mortality, whereas in the analysis by Naal et al, only serum chloride at 6‐month follow‐up from diagnosis was predictive of increased risk of mortality.9 Some possible explanations for these discrepancies are differences in age and PAH etiology in the hypochloremia groups. First, hypochloremic patients in our study cohort are slightly younger (54±14) when compared with hypochloremic patients in the Naal et al study (58±18).9 This may have led to fewer comorbid conditions, which may explain why the mean pulmonary capillary wedge pressure (9±2 mm Hg) was lower in our study cohort compared with theirs (11±4 mm Hg).9 More importantly, we studied a different PAH population because we included all PAH patients not just idiopathic PAH and heritable PAH. Finally, our cohort had a significantly larger number of patients (n=475 versus 277), which increases the power of our statistical analysis. Despite the differences between the 2 studies, both demonstrate that hypochloremia is an independent predictor of mortality in PAH patients, and these reproducible findings suggest that more work is needed to understand the mechanistic underpinnings of this observation.
It is unknown whether hypochloremia is simply a biomarker of impaired RV function, renal impairment, or loop diuretic use or a direct contributor to the pathology. It is possible that serum chloride is a surrogate for diuretic dose, which is associated with mortality in patients with left heart failure.19, 20 However, there are cellular mechanisms by which hypochloremia could promote disease. For example, the family of With No Lysine (WNK) kinases are intracellular chloride sensors that mediate signal transduction of hypochloremia.21 WNK proteins are activated by a process of autophosphorylation, which is inhibited by chloride; conversely hypochloremia activates WNKs.22 The role of WNKs in the heart is not well studied, but WNK1 ablation causes embryonic lethality due to altered cardiac development.23, 24 Moreover, WNK1 signaling promotes membrane localization of the glucose transporters Glut125 and Glut4,26 and both of these glucose transporters are upregulated in animal models of right ventricular pressure overload.27, 28, 29, 30 Thus, hypochloremia might affect RV metabolism and/or function via WNK1. Furthermore, hypochloremia is mechanistically linked to diuretic resistance,31 which could worsen right heart failure due to volume overload. Together, these observations suggest that hypochloremia may not be just a marker of disease severity and diuretic use. However, mechanistic studies are needed to definitively assess this possibility.
Our results add to the growing body of literature that demonstrates that serum chloride levels are important for predicting poor outcomes in cardiovascular disease. Hypochloremia was first shown to predict mortality in acute decompensated systolic left heart failure independent of serum sodium.6 This result has been validated in multiple other studies in both the acute decompensated settings and chronic heart failure settings.32, 33, 34, 35 Evidently, serum chloride more accurately predicts outcomes in diverse left heart failure groups than serum sodium, and future studies are required to determine if serum chloride supplementation could be a novel therapy for left ventricular systolic heart failure or right heart failure due to PAH.
Prognostication plays an important role in the management of PAH, as it remains an incurable disease with limited median survival of 5 to 7 years1 with recurrent hospitalizations.36 Currently, there are several models that are available to assist clinicians in risk assessment of patients with PAH.37 These risk‐prediction models are routinely used in the Western countries in the initiation as well as escalation of PAH‐specific vasodilator therapies.37 However, these risk‐prediction models involve extensive testing including laboratory investigations, ECGs, and right heart catheterization.37 In resource‐limited countries, application of these risk‐prediction models is limited by their cost. The new Minnesota noninvasive model that we propose predicts prognosis similar to the French noninvasive model but has the advantage of using variables that are widely available and inexpensive, including the presence of hypochloremia, 6MWD, and functional class. Thus, it could be a cost‐effective risk‐prediction tool for PAH, especially in resource‐limited regions.
Limitations
Our study has important limitations that must be acknowledged. First, we combined patients from 2 different PH registries (University of Minnesota PH registry and VUMC PH registry). Thus, our results may have been influenced by the differences in the registry design and practice patterns. However, this was done to create a true multicentered approach and to accommodate for minor differences in treatment practices to evaluate the widespread utility of serum chloride for PAH prognostication. Moreover, we defined hypochloremia by dividing patients into tertiles rather than using the definition employed in heart failure (<96 mmol/L),6 as the number of PAH patients with serum chloride that low was small in our study cohort (n=25). We identified a value of ≤101 mEq/L, which was close to the value used by Naal et al (≤100 mEq/L). However, both of these values are actually within the normal range of chloride and higher than the cutoff used in heart failure. On multivariable analysis, serum chloride was no longer an independent predictor of mortality when we included mean right atrial pressure. This is likely due to the significant collinearity between serum chloride levels and the mean right atrial pressure (r=−0.142, P<0.01). However, our data do not fully address the question whether there is a cause‐and‐effect relationship between serum chloride and worse right ventricular function. Finally, these results may not extrapolate to resource‐limited regions of the world because the study was conducted in 2 large tertiary referral centers, as the underlying drivers of PAH may be different in resource‐limited regions of the world and in North America. Finally, we did not perform nonlinear assessments of serum chloride and clinical variables.
Conclusions
Hypochloremia is associated with markers of RV failure and is a noninvasive predictor of mortality in PAH independent of age, sex, serum sodium, renal function, and diuretic use. Serum chloride may be helpful to prognosticate PAH patients, especially in countries with limited access to resources and allow for better triage of expensive PAH therapy.
Sources of Funding
Prins is funded by NIH K08 HL140100, the Cardiovascular Medical Research and Education Fund, and the United Therapeutics Jenesis Award. Archer is funded by Canada Foundation for Innovation (229252 and 33012), a Tier 1 Canada Research Chair in Mitochondrial Dynamics and Translational Medicine (950‐229252), the Queens Cardiopulmonary Unit (QCPU), and a grant from the William J Henderson Foundation. Brittain is funded by NIH R34 HL136989 and R01s HL128983 and HL146588. Thenappan is funded by AHA Scientist Development Grant (15SDG25560048). Bajaj is supported by American College of Cardiology Presidential Career Development Award, the Walter B. Frommeyer, Junior Fellowship in Investigative Medicine, and National Center for Advancing Translational Research of the National Institutes of Health (UL1TR001417). Prisco is funded by NIH T32 HL144472.
Disclosures
Prins has served on an advisory board for Actelion. Brittain has served on an advisory board for Bayer. Thenappan has served on an advisory board for Actelion and Gilead. The remaining authors have no disclosures to report.
Supporting information
Figure S1. Correlation between serum chloride and serum sodium.
Figure S2. Both female and male PAH patients with low chloride have increased mortality.
Figure S3. Forest plots of unadjusted and adjusted hazard ratios of lowest‐chloride‐tertile patients compared with middle‐ and highest‐chloride‐tertile patients.
(J Am Heart Assoc. 2020;9:e015221 DOI: 10.1161/JAHA.119.015221.)
References
- 1. Thenappan T, Ormiston ML, Ryan JJ, Archer SL. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ. 2018;360:j5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prins KW, Thenappan T. World Health Organization Group I pulmonary hypertension: epidemiology and pathophysiology. Cardiol Clin. 2016;34:363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gidwani S, Nair A. The burden of pulmonary hypertension in resource‐limited settings. Glob Heart. 2014;9:297–310. [DOI] [PubMed] [Google Scholar]
- 4. Galiè N, Channick RN, Frantz RP, Grünig E, Jing ZC, Moiseeva O, Preston IR, Pulido T, Safdar Z, Tamura Y, McLaughlin VV. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53:1801889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Forfia PR, Mathai SC, Fisher MR, Housten‐Harris T, Hemnes AR, Champion HC, Girgis RE, Hassoun PM. Hyponatremia predicts right heart failure and poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;177:1364–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grodin JL, Simon J, Hachamovitch R, Wu Y, Jackson G, Halkar M, Starling RC, Testani JM, Tang WH. Prognostic role of serum chloride levels in acute decompensated heart failure. J Am Coll Cardiol. 2015;66:659–666. [DOI] [PubMed] [Google Scholar]
- 7. Grodin JL, Verbrugge FH, Ellis SG, Mullens W, Testani JM, Tang WH. Importance of abnormal chloride homeostasis in stable chronic heart failure. Circ Heart Fail. 2016;9:e002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cuthbert JJ, Pellicori P, Rigby A, Pan D, Kazmi S, Shah P, Clark AL. Low serum chloride in patients with chronic heart failure: clinical associations and prognostic significance. Eur J Heart Fail. 2018;20:1426–1435. [DOI] [PubMed] [Google Scholar]
- 9. Naal T, Abuhalimeh B, Khirfan G, Dweik RA, Tang WHW, Tonelli AR. Serum chloride levels track with survival in patients with pulmonary arterial hypertension. Chest. 2018;154:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prins KW, Weir EK, Archer SL, Markowitz J, Rose L, Pritzker M, Madlon‐Kay R, Thenappan T. Pulmonary pulse wave transit time is associated with right ventricular‐pulmonary artery coupling in pulmonary arterial hypertension. Pulm Circ. 2016;6:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thayer TE, Huang S, Levinson RT, Farber‐Eger E, Assad TR, Huston JH, Mosley JD, Wells QS, Brittain EL. Unbiased phenome‐wide association studies of red cell distribution width identifies key associations with pulmonary hypertension. Ann Am Thorac Soc. 2019;16:589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Assad TR, Hemnes AR, Larkin EK, Glazer AM, Xu M, Wells QS, Farber‐Eger EH, Sheng Q, Shyr Y, Harrell FE, Newman JH, Brittain EL. Clinical and biological insights into combined post‐ and pre‐capillary pulmonary hypertension. J Am Coll Cardiol. 2016;68:2525–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mohammed SF, Hussain I, AbouEzzeddine OF, Abou Ezzeddine OF, Takahama H, Kwon SH, Forfia P, Roger VL, Redfield MM. Right ventricular function in heart failure with preserved ejection fraction: a community‐based study. Circulation. 2014;130:2310–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol. 2006;47:799–803. [DOI] [PubMed] [Google Scholar]
- 16. Boucly A, Weatherald J, Savale L, Jaïs X, Cottin V, Prevot G, Picard F, de Groote P, Jevnikar M, Bergot E, Chaouat A, Chabanne C, Bourdin A, Parent F, Montani D, Simonneau G, Humbert M, Sitbon O. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J. 2017;50:1700889. [DOI] [PubMed] [Google Scholar]
- 17. Pencina MJ, D'Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172; discussion 207‐112 [DOI] [PubMed] [Google Scholar]
- 18. Pencina MJ, D'Agostino RB, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Valente MA, Voors AA, Damman K, Van Veldhuisen DJ, Massie BM, O'Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison B, Cleland JG, Givertz MM, Bloomfield DM, Fiuzat M, Dittrich HC, Hillege HL. Diuretic response in acute heart failure: clinical characteristics and prognostic significance. Eur Heart J. 2014;35:1284–1293. [DOI] [PubMed] [Google Scholar]
- 20. Hasselblad V, Gattis Stough W, Shah MR, Lokhnygina Y, O'Connor CM, Califf RM, Adams KF. Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. Eur J Heart Fail. 2007;9:1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shekarabi M, Zhang J, Khanna AR, Ellison DH, Delpire E, Kahle KT. WNK kinase signaling in ion homeostasis and human disease. Cell Metab. 2017;25:285–299. [DOI] [PubMed] [Google Scholar]
- 22. Piala AT, Moon TM, Akella R, He H, Cobb MH, Goldsmith EJ. Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal. 2014;7:ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xie J, Yoon J, Yang SS, Lin SH, Huang CL. WNK1 protein kinase regulates embryonic cardiovascular development through the OSR1 signaling cascade. J Biol Chem. 2013;288:8566–8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zambrowicz BP, Abuin A, Ramirez‐Solis R, Richter LJ, Piggott J, BeltrandelRio H, Buxton EC, Edwards J, Finch RA, Friddle CJ, Gupta A, Hansen G, Hu Y, Huang W, Jaing C, Key BW, Kipp P, Kohlhauff B, Ma ZQ, Markesich D, Payne R, Potter DG, Qian N, Shaw J, Schrick J, Shi ZZ, Sparks MJ, Van Sligtenhorst I, Vogel P, Walke W, Xu N, Zhu Q, Person C, Sands AT. Wnk1 kinase deficiency lowers blood pressure in mice: a gene‐trap screen to identify potential targets for therapeutic intervention. Proc Natl Acad Sci USA. 2003;100:14109–14114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mendes AI, Matos P, Moniz S, Jordan P. Protein kinase WNK1 promotes cell surface expression of glucose transporter GLUT1 by regulating a Tre‐2/USP6‐BUB2‐Cdc16 domain family member 4 (TBC1D4)‐Rab8A complex. J Biol Chem. 2010;285:39117–39126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim JH, Kim H, Hwang KH, Chang JS, Park KS, Cha SK, Kong ID. WNK1 kinase is essential for insulin‐stimulated GLUT4 trafficking in skeletal muscle. FEBS Open Bio. 2018;8:1866–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fang YH, Piao L, Hong Z, Toth PT, Marsboom G, Bache‐Wiig P, Rehman J, Archer SL. Therapeutic inhibition of fatty acid oxidation in right ventricular hypertrophy: exploiting Randle's cycle. J Mol Med (Berl). 2012;90:31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Piao L, Fang YH, Cadete VJ, Wietholt C, Urboniene D, Toth PT, Marsboom G, Zhang HJ, Haber I, Rehman J, Lopaschuk GD, Archer SL. The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: resuscitating the hibernating right ventricle. J Mol Med (Berl). 2010;88:47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Broderick TL, King TM. Upregulation of GLUT‐4 in right ventricle of rats with monocrotaline‐induced pulmonary hypertension. Med Sci Monit. 2008;14:BR261–BR264. [PubMed] [Google Scholar]
- 30. Paulin R, Sutendra G, Gurtu V, Dromparis P, Haromy A, Provencher S, Bonnet S, Michelakis ED. A miR‐208‐Mef2 axis drives the decompensation of right ventricular function in pulmonary hypertension. Circ Res. 2015;116:56–69. [DOI] [PubMed] [Google Scholar]
- 31. Hanberg JS, Rao V, Ter Maaten JM, Laur O, Brisco MA, Perry Wilson F, Grodin JL, Assefa M, Samuel Broughton J, Planavsky NJ, Ahmad T, Bellumkonda L, Tang WH, Parikh CR, Testani JM. Hypochloremia and diuretic resistance in heart failure: mechanistic insights. Circ Heart Fail. 2016;9:e003180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ter Maaten JM, Damman K, Hanberg JS, Givertz MM, Metra M, O'Connor CM, Teerlink JR, Ponikowski P, Cotter G, Davison B, Cleland JG, Bloomfield DM, Hillege HL, van Veldhuisen DJ, Voors AA, Testani JM. Hypochloremia, diuretic resistance, and outcome in patients with acute heart failure. Circ Heart Fail. 2016;9:e003109. [DOI] [PubMed] [Google Scholar]
- 33. Grodin JL, Testani JM, Pandey A, Sambandam K, Drazner MH, Fang JC, Tang WHW. Perturbations in serum chloride homeostasis in heart failure with preserved ejection fraction: insights from TOPCAT. Eur J Heart Fail. 2018;20:1436–1443. [DOI] [PubMed] [Google Scholar]
- 34. Grodin JL, Sun JL, Anstrom KJ, Chen HH, Starling RC, Testani JM, Tang WH. Implications of serum chloride homeostasis in acute heart failure (from ROSE‐AHF). Am J Cardiol. 2017;119:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Testani JM, Hanberg JS, Arroyo JP, Brisco MA, Ter Maaten JM, Wilson FP, Bellumkonda L, Jacoby D, Tang WH, Parikh CR. Hypochloraemia is strongly and independently associated with mortality in patients with chronic heart failure. Eur J Heart Fail. 2016;18:660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anand V, Roy SS, Archer SL, Weir EK, Garg SK, Duval S, Thenappan T. Trends and outcomes of pulmonary arterial hypertension‐related hospitalizations in the United States: analysis of the nationwide inpatient sample database from 2001 through 2012. JAMA Cardiol. 2016;1:1021–1029. [DOI] [PubMed] [Google Scholar]
- 37. Benza RL, Farber HW, Selej M, Gomberg‐Maitland M. Assessing risk in pulmonary arterial hypertension: what we know, what we don't. Eur Respir J. 2017;50:1701353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Correlation between serum chloride and serum sodium.
Figure S2. Both female and male PAH patients with low chloride have increased mortality.
Figure S3. Forest plots of unadjusted and adjusted hazard ratios of lowest‐chloride‐tertile patients compared with middle‐ and highest‐chloride‐tertile patients.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.


