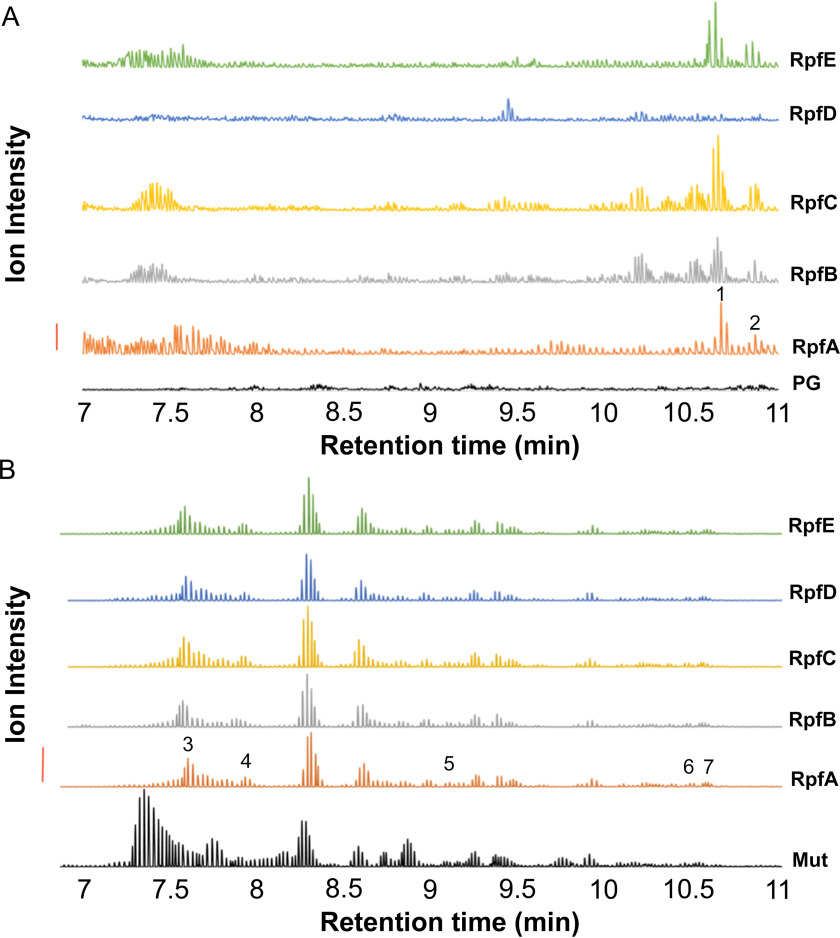
Figure 2.
Characterization of RpfA-E as endo-lytic transglycosylases by LC-Q-TOF MS analysis of their reaction products. Samples of M. luteus peptidoglycan suspended in [18O]H2O to a final concentration of 1.4 mg/ml were incubated separately with 1 nmol Rpf or 1.1 nmol mutanolysin (positive control). After incubation at 37 °C for 9.5 h, soluble reaction products were separated from insoluble material by centrifugation. The insoluble peptidoglycan pellets from the Rpf digestions were washed with water and then resuspended in 0.1 mm potassium phosphate buffer, pH 6.2, for solubilization by 1.1 μm mutanolysin. Soluble muropeptides from this secondary digestion were recovered by centrifugation. Each soluble and secondary-soluble fraction was subjected to LC-Q-TOF MS analysis. A, analysis of soluble fraction from peptidoglycan alone (PG), and reactions with 1 nmol RpfA–E, as indicated. B, analysis of insoluble products following secondary mutanolysin digestion from reaction with 1.1 μmol mutanolysin (Mut; positive control); or 1 nmol RpfA–E, as indicated. The identities of the numbered muropeptide fractions are listed in Table 2. The solid vertical bar to the left denotes 10,000 and 200,000 intensity units, for panels A and B, respectively.