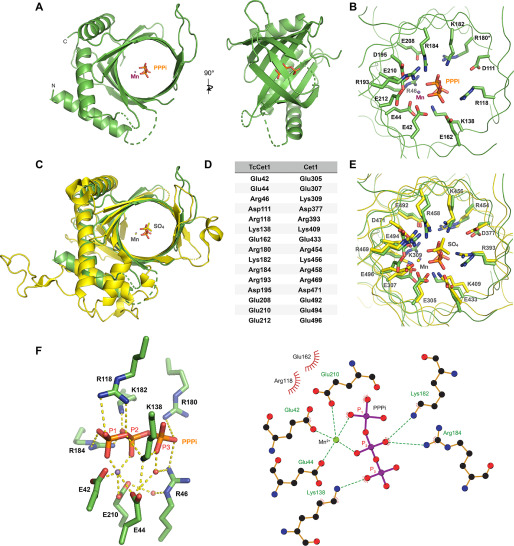
Figure 2.
Structure of TcCet1(18–243 Δ55–75) in complex with tripolyphosphate in the active site. A, crystal structure of TcCet1(18–243 Δ55–75) is depicted as a cartoon model. PPPi is shown as sticks, and manganese is shown as a magenta sphere. B, cross-section of the triphosphate tunnel of TcCet1(18–243 Δ55–75). C, the structure of TcCet1(18–243 Δ55–75) colored in green was superimposed on a yeast Cet1(210-549) colored in yellow (18) (Protein Data Bank entry 1D8H). The sulfate and manganese in Cet1(210-549) are depicted as yellow sticks and a yellow sphere, respectively. D, the amino acids of TcCet1 that correspond to active site residues of Cet1, as judged from the structural comparison. The Arg180 side chain was not visible in Mn·PPPi–bound structure and therefore was modeled from the manganese-bound structure. E, superimposition of triphosphate tunnel of TcCet1(18–243 Δ55–75) and Cet1(210-549). The image was prepared with PyMOL. F, the figure highlights the network of bonding interactions that coordinate PPPi, manganese, and two water molecules (red sphere) in TcCet1(18–243 Δ55–75). The images were prepared with PyMOL and LigPlus.