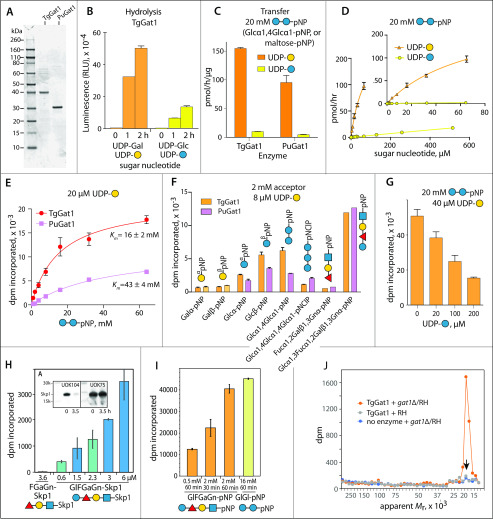
Figure 4.
Gat1 preferentially galactosylates the Skp1 glycan in vitro. A, recombinantly expressed and purified preparations of TgGat1 and PuGat1 were analyzed by SDS-PAGE and staining with Coomassie blue. B, temporal dependence of UDP-Gal and UDP-Glc hydrolysis. The averages and S.D. of three technical replicates are shown. A similar profile was observed with a different enzyme concentration. See Fig. S9E for a trial with higher enzyme concentrations. C, transferase activity utilizing 8 μm UDP-Gal or UDP-Glc toward 20 mm Glcα1,4Glcα-pNP (maltose-pNP) for TgGat1 and PuGat1. The averages and S.D. of two technical replicates are shown; similar profiles were in two independent assays with a different TgGat1 preparation. D, UDP-Gal and UDP-Glc concentration dependence of TgGat1 transferase activity toward 20 mm maltose-pNP. The averages and S.D. of two technical replicates are shown, and an independent trial with TgGat1 and PuGat1 against UDP-Gal is shown in Fig. S9F. E, maltose-pNP concentration dependence of TgGat1 and PuGat1 transferase activity from 20 μm UDP-Gal. The averages and S.D. of two technical replicates are shown. F, relative Gal-transferase activity of TgGat1 and PuGat1 toward different acceptors. The averages and S.D. of three technical replicates are shown. Similar results were obtained in three independent trials. G, effect of UDP-Glc concentration on the Gal-transferase activity of TgGat1. Reactions were incubated for 1 h. The averages and S.D. of two technical replicates are shown. H, Gal-transferase activity of TgGaT1 toward varied concentrations of GlFGaGn-Skp1, in the presence of 40 μm UDP-Gal (1 µCi) after a 1-h incubation. Data from independent preparations of TgSkp1 are colored in different shades. FGaGn-Skp1 is included for comparison. Error bars, S.D. of duplicate measurements. Inset, Western blots of the Skp1 preparations used, where FGaGn-Skp1, which is recognized specifically by pAb UOK104, is largely converted in a 3.5-h reaction using Glt1 and UDP-Glc to GlFGaGn-Skp1, which is recognized only by the pan-specific pAb UOK75. I, reactions with synthetic oligosaccharides conjugated to pNP were conducted in parallel using the same conditions. J, biochemical complementation to detect Gat1 substrates. Desalted S100 extracts of RH and gat1Δ/RH were reacted with recombinant Gat1 in the presence of UDP-[3H]Gal, and the product of the reaction was separated on an SDS-polyacrylamide gel, which was sliced into 40 bands for liquid scintillation counting. The migration position of Skp1 is marked with an arrow. See Fig. S9 (H and I) for trials using different strains.