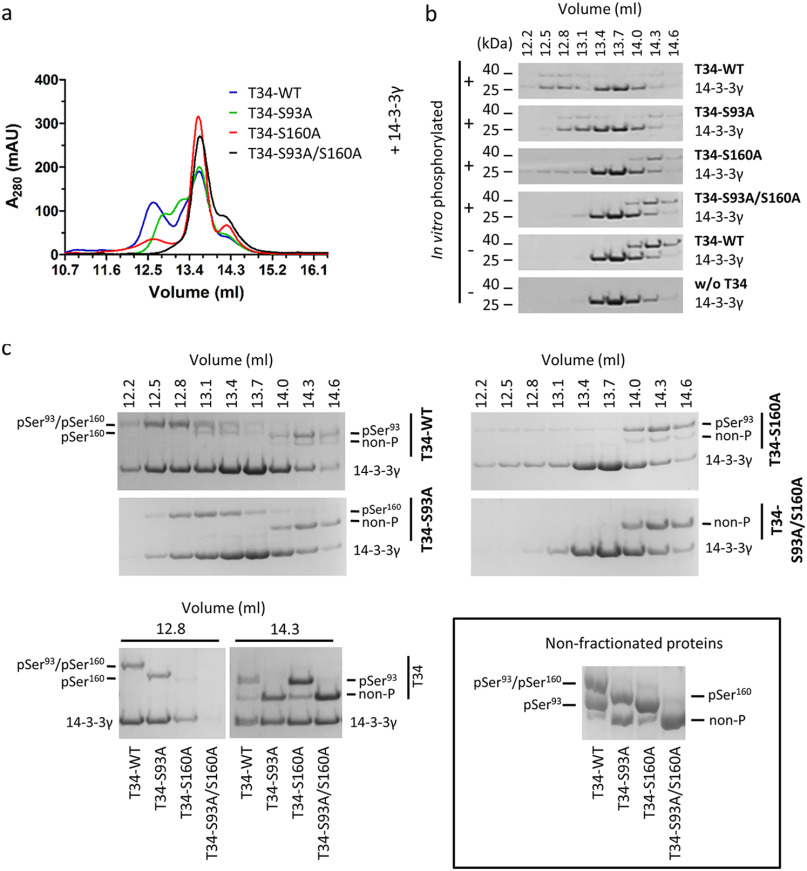
Figure 3.
Ser160 phosphorylation by PKA is crucial for 14-3-3 dimers binding. a, PKA-phosphorylated TOMM34 variants (T34, 35 μm) were preincubated with 14-3-3γ protein (70 μm) for 30 min at 21 °C before separation by analytical SEC. b, indicated fractions from (a) and from SEC separations of nonphosphorylated TOMM34/14-3-3γ (see Fig. 2 and Fig. S1) were analyzed by gel electrophoresis and Coomassie staining. c, indicated fractions from (a) and (b) as well as nonfractioned proteins were separated in Phos-tag gels retarding the migration of phosphorylated proteins and stained by Coomassie (45). MS-identified phosphoforms of TOMM34 in 14-3-3γ-bound (elution volume, 12.8 ml) and unbound (elution volume, 14.3 ml) fractions are indicated.