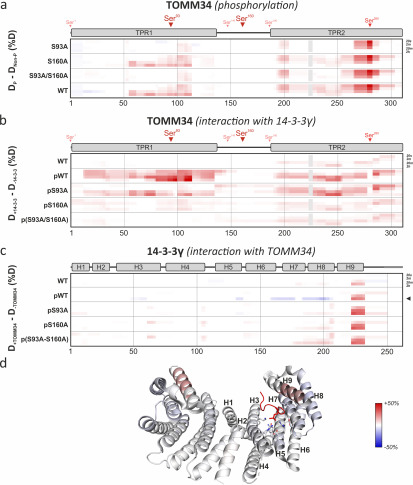
Figure 4.
Phosphorylation induces destabilization of TOMM34 secondary structure augmented by 14-3-3γ binding. Time resolved deuteration level differences between selected experimental conditions (indicated on the left side) presented in the form of heat maps. Horizontal gray lines separate individual experimental conditions. Within each experimental condition, the four rows correspond to exchange times (20 s, 2 min, 20 min, and 2 h from top to bottom), indicated on the right side. The vertical gray lines mark sequence positions (spaced by 50 amino acids). a, difference between phosphorylated (p) TOMM34 WT, S93A, S160A, and/or S93A/S160A proteins and their nonphosphorylated forms. TOMM34 domain structure is depicted above the heat map with PKA-modified sites indicated by arrowheads (see Fig. 1). Regions in gray boxes were not covered. b, differences between nonphosphorylated TOMM34 WT and phosphorylated (p) TOMM34 WT, S93A, S160A, and/or S93A/S160A proteins in the presence and absence of 14-3-3γ protein. c, differences between 14-3-3γ protein incubated in the presence or absence of nonphosphorylated TOMM34 WT and PKA-phosphorylated (p) TOMM34 WT, S93A, S160A, and/or S93A/S160A. 14-3-3γ domain structure is depicted above the heat map. d, deuteration level differences between 14-3-3γ protein incubated with and without PKA-phosphorylated TOMM34 protein after 2 h of deuteration (indicated in c by an arrowhead) mapped on the structure of 14-3-3γ dimer in complex with phosphopeptide (in red) (PBD code 6A5S; Ref. 50). Residues involved in phosphoserine interaction (Lys50, Arg57, Arg132, Tyr133, Glu136, Leu177, and Glu185) and α-helices are highlighted in one of 14-3-3γ protomers.