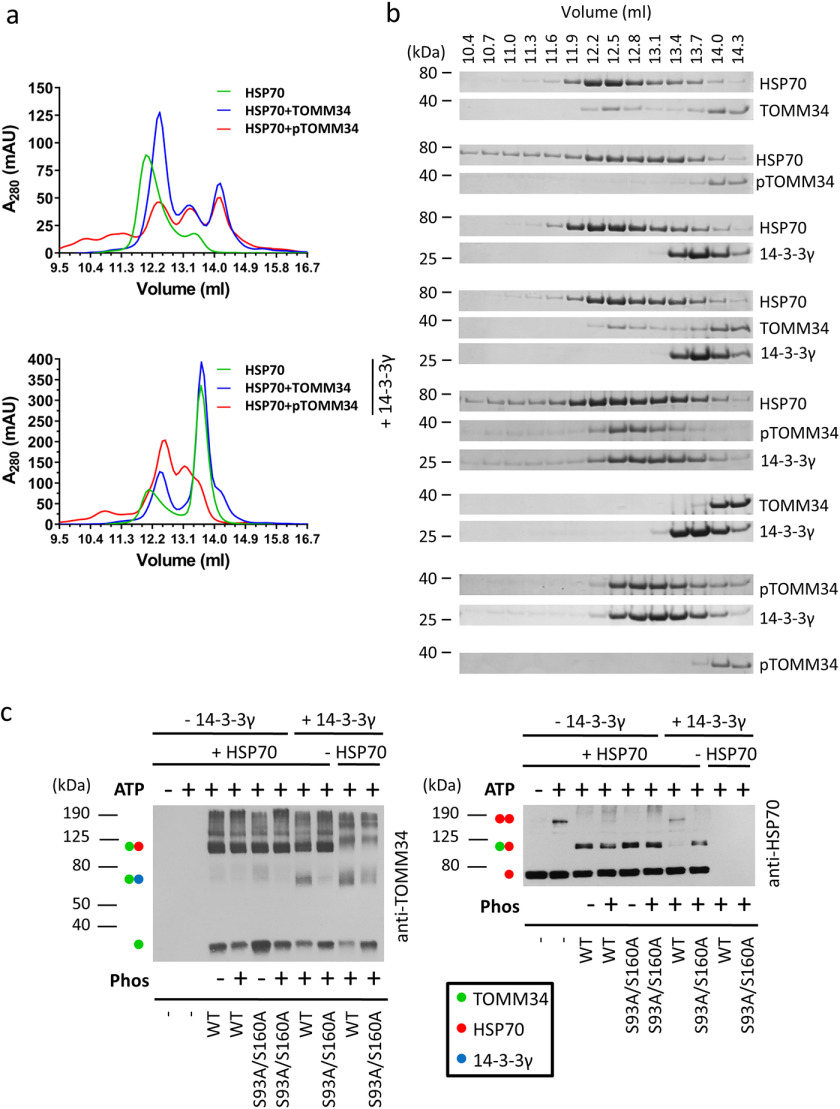
Figure 7.
Binding of pTOMM34 to 14-3-3γ dimer prevents disruption of ATP-dependent HSP70 dimer. a, phosphorylated/nonphosphorylated TOMM34 WT preincubated with/without 14-3-3γ (30 min, 21 °C) was mixed with HSP70 in the presence of 0.2 mm ATP and incubated 20 min at 21 °C before separation by analytical SEC. The final concentrations of TOMM34, 14-3-3γ, and HSP70 in the protein mixtures were 35, 70, and 35 μm, respectively. b, indicated fractions from (a) were analyzed by gel electrophoresis and Coomassie staining. c, intact or PKA-phosphorylated WT and/or S93A/S160A (final concentration, 60 μm) proteins were mixed with 14-3-3γ (final concentration, 120 μm) or buffer. HSP70 protein (60 μm) preincubated with or without ATP (0.4 mm) was added to TOMM34/14-3-3γ samples in 1:1 ratio, and the mixture was chemically cross-linked by glutaraldehyde addition. The reactions were stopped after 10 min with Tris, pH 8, and separated by SDS-PAGE, blotted, and probed with anti-TOMM34 and HSP70 antibodies. Molecular mass markers and captured protein assemblies are indicated by numbers and dots, respectively.