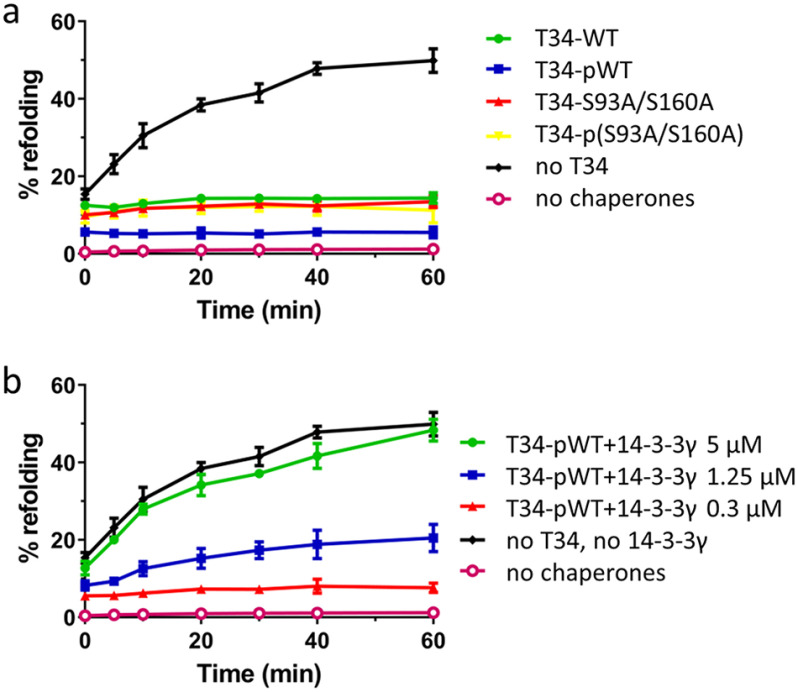
Figure 8.
Sequestration of pTOMM34 by 14-3-3γ eliminates TOMM34's inhibitory role in HSP70-mediated refolding. a, firefly luciferase incubated with HSP70 (1 μm), HSP40 (2 μm), and BAG1 (0.5 μm) proteins was thermally denatured at 42 °C for 30 min in the presence of 5 μm nonphosphorylated/PKA-phosphorylated TOMM34 WT and S93A/S160A proteins. The kinetics of luciferase reactivation was measured after shifting the reaction temperature to 37 °C. b, firefly luciferase denatured as in (a) was refolded in the presence of 5 μm PKA-phosphorylated TOMM34 WT protein and increasing concentrations of 14-3-3γ dimer (0.3, 1.25, and 5 μm). The signal from samples with native luciferase was set as 100%. As negative controls, we measured the luciferase activity of denatured luciferase only. Error bars represent S.D.; n = 3 independent experiments.