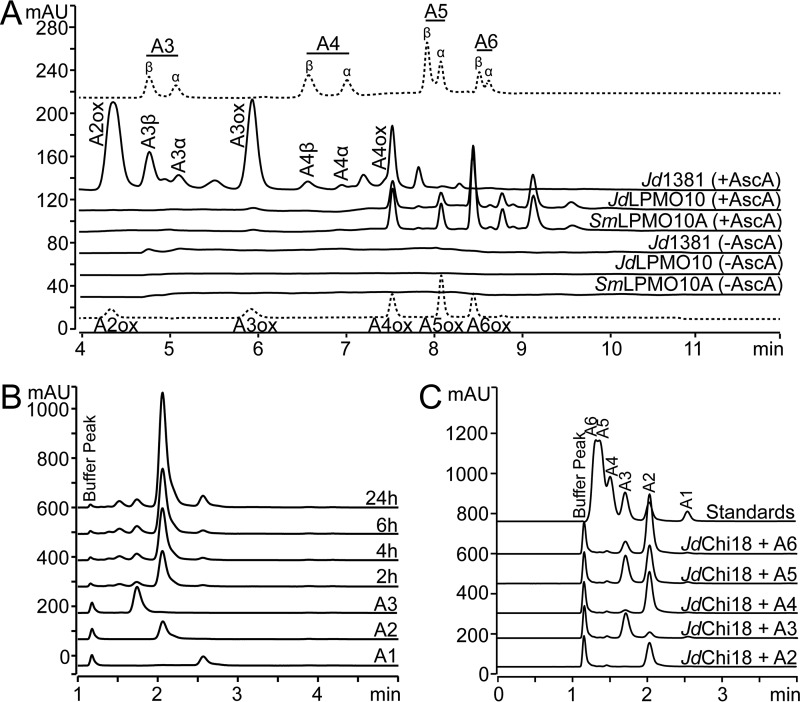
Figure 3.
Catalytic activity of Jd1381 and its individual domains. Chito-oligosaccharides are labeled as “An,” where n indicates the number of sugars; ox indicates oxidation. A, soluble oxidized products released by Jd1381 or JdLPMO10 or SmLPMO10A from β-chitin, analyzed by HPLC using a HILIC column. The reactions contained 1 μm Cu(II) saturated enzyme and 10 g/liter substrate in 20 mm BisTris, pH 6.0, with or without 1 mm AscA. The reactions were incubated for 24 h at 40 °C, with shaking at 1000 rpm. This experiment was repeated three times, with similar results. The upper and lower chromatograms (dotted lines) show standard mixtures containing native (A3–A6) and oxidized (A2ox–A6ox) chito-oligosaccharides, respectively. Native oligomers appear as double peaks because the α- and β-anomers are separated, as indicated. The small peaks eluting in between A3 and A3ox, between A4 and A4ox and after A5 are products of unknown nature. Note that by far the most dominant soluble product generated in the reactions with Jd1381 (regardless of the presence of AscA) was the nonoxidized dimer (chitobiose, A2, GlcNAc2), which is not visible in A and was analyzed using another chromatographic method, as shown in B. B, nonoxidized products generated from β-chitin by Jd1381, analyzed by ion-exclusion chromatography. The reaction conditions were as described above, with added ascorbic acid. The compounds eluting prior to GlcNAc2 are oxidized products. C, products generated by JdChi18 from soluble chito-oligosaccharides in reaction mixtures containing 0.5 mm substrate and 10 nm JdChi18 in 20 mm BisTris, pH 6.0, after incubation for 5 h at 40 °C. The standard mixture contained 50 μm of A1–A6. Fig. S3 shows that full-length Jd1381 and JdChi18 are equally active toward A5.