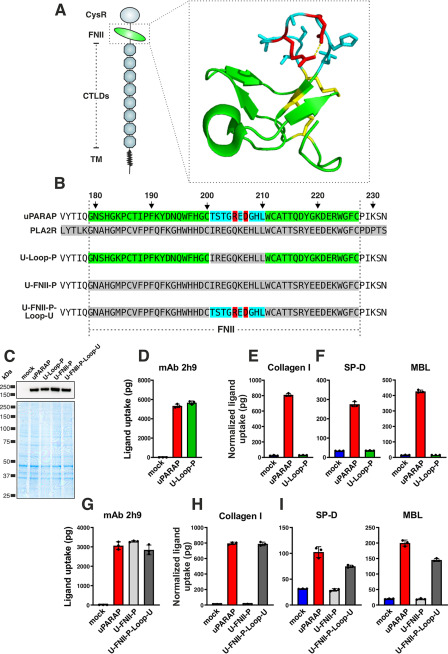
Figure 1.
A protruding loop in the FNII domain is essential for uPARAP-mediated uptake of collectins and collagen. A, overview of domains in uPARAP and the crystal structure of uPARAP's FNII domain (24). CysR, cysteine rich; TM, transmembrane region. The protruding loop is shown with side chains as sticks (residues in blue). A salt bridge within the loop, formed between Arg-205 and Asp-207 (residues in red), is represented by a dotted yellow line. Cysteines and disulfide bonds are highlighted (residues in yellow). B, alignment of FNII domain sequences from uPARAP, PLA2R1, and three uPARAP mutant receptors: U-loop-P, uPARAP with residues Thr-201–Leu-210 replaced by the corresponding residues from PLA2R1; U-FNII-P, uPARAP with complete FNII domain (Gly-179–Cys-227) replaced by FNII residues from PLA2R1; U-FNII-P-loop-U, the U-FNII-P mutant receptor with uPARAP residues Thr-201–Leu-210 reintroduced. Amino acid numbering follows the uPARAP sequence (accession no. NP_032652.3). C, western blotting analysis for the detection of uPARAP in mock transfected CHO-K1 cells and cells transfected to express uPARAP, U-loop-P, U-FNII-P, or U-FNII-P-loop-U (top panel). Nonreducing SDS-PAGE and Coomassie Brilliant Blue staining of protein lysates was included as loading controls for the western blotting (bottom panel). D–I, assay for uptake of radiolabeled mAb 2h9 (D and G), collagen type I (E and H), and SP-D and MBL (F and I) by mock-transfected CHO-K1 cells and cells transfected to express uPARAP or the indicated mutants. Analysis was performed in triplicate. The uptake of collagen type I, SP-D, and MBL was normalized to the uptake of anti-uPARAP antibody 2h9 to adjust for minor differences in the receptors' expression levels (see “Experimental procedures”). Data are presented as mean ± S.D. with individual data points also shown. Analysis was performed in triplicate.