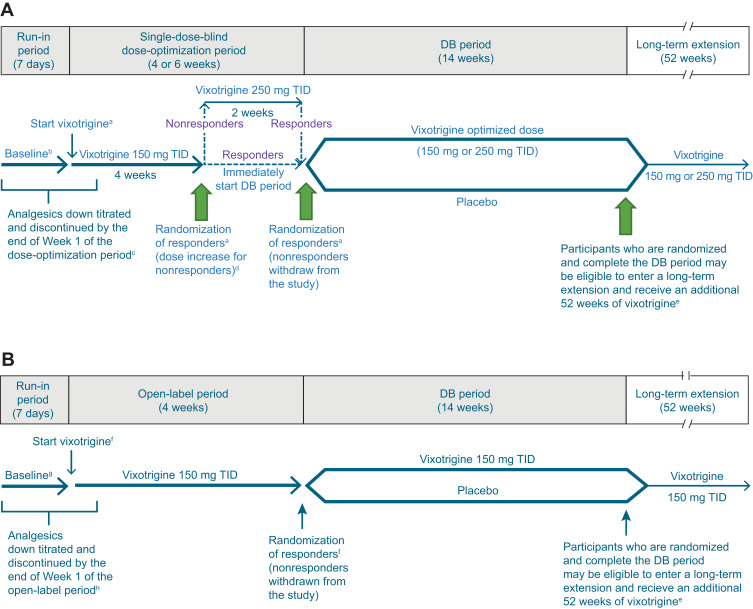
Figure 1.
Study design for (A) Study 1 and (B) Study 2.
Notes: Responders are defined as participants with ≥30% reduction in mean pain score from run-in period baseline to the last week of the dose-optimization/open-label period. aParticipants must meet all eligibility criteria on Day 1 to enter the dose-optimization period and all randomization criteria at Week 4 (Day 29) or Week 6 (Day 43) to be randomized to DB treatment. bFor efficacy endpoints based on daily participant diary (pain score, worst pain score, and number of paroxysms), baseline will be defined as the means of the diary data recorded over the 7 days preceding the first dose of study treatment in the dose-optimization period. For other efficacy endpoints, baseline will be the last measurement before the first dose of study treatment. cParticipants taking >1 TN medication at study entry will be required to gradually titrate down and discontinue their medications so that they are receiving no more than 1 TN medication at the start of the dose-optimization period. The remaining TN medication should be at a low enough dose at the start of the dose-optimization period so that it can be safely stopped by the end of Week 1. Participants taking carbamazepine or oxcarbazepine will be required to reduce their dose by the start of the dose-optimization period and will take their last dose by Day 7, prior to the start of Week 2 of the dose-optimization period. dThe increase in dose at the end of Week 4 for nonresponders will occur only if participants have recorded their pain score in the electronic diary on ≥5 of the last 7 days of the dose-optimization period; participants who are noncompliant with the electronic diary will be withdrawn from the study. eIncludes participants who discontinue DB study treatment for reasons other than adverse events but remain in the study and complete the DB period through Week 14. Participants who discontinue DB study treatment and withdraw from the study and participants who exceed dosing limits for acetaminophen/paracetamol, pregabalin, or immediate-release oxycodone during the DB period will not be eligible for the long-term extension. fParticipants must meet all eligibility criteria on Day 1 to enter the open-label period and all randomization criteria at Week 4 (Day 29) to be randomized to DB treatment. gFor efficacy endpoints based on daily participant diary (pain score, worst pain score, and number of paroxysms), baseline will be defined as the means of the diary data recorded over the 7 days preceding the first dose of study treatment in the open-label period. For other efficacy endpoints, baseline will be the last measurement before the first dose of study treatment. hParticipants taking >1 TN medication at study entry will be required to gradually titrate down and discontinue their medications so that they are receiving no more than 1 TN medication at the start of the open-label period. The remaining TN medication should be at a low enough dose at the start of the open-label period so that it can be safely stopped by the end of Week 1. Participants taking carbamazepine or oxcarbazepine will be required to reduce their dose by the start of the open-label period and will take their last dose by Day 7, prior to the start of Week 2 of the open-label period.
Abbreviations: DB, double-blind; TID, 3 times daily; TN, trigeminal neuralgia.