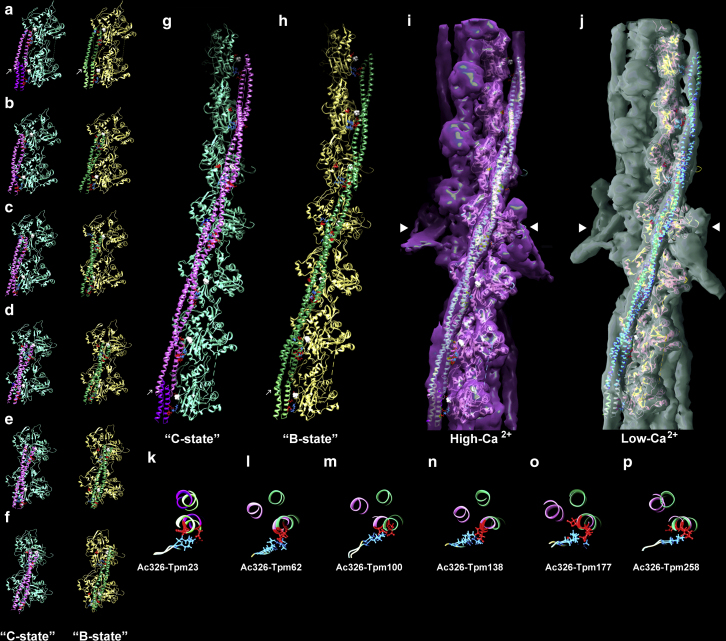
Figure 1.
Docking tropomyosin segments to F-actin. (a–f) Highest scoring αβα-zone tropomyosin segments dock to F-actin in one of two modes, viz. those fulfilling a C-state pose (magenta on cyan-colored actin) and those with a B-state-pose (green on tan-colored actin). Individual docking poses on actin are illustrated by (a) the overlapping domain, i.e., β6α7β7α1β1α2, or by (b–f) different intrachain segments (b) α6β6α7, (c) α5β5α6, (d) α4β4α5, (e) α3β3α4, and (f) α2β2α3. Ribbon diagrams are shown with side chains on actin residues Asp311, Lys326, and Lys328 (red, blue, blue); side chains of tropomyosin residues that interact with actin are also indicated. Side chains of actin’s Pro333, which demarcates the inner and outer domains of actin, are highlighted with white spheres. Matching common actin subunits in the segments shown above were superposed to generate composite “single-stranded” actin-tropomyosin filaments (g and h), and then the composite filaments were fitted within respective high- and low-Ca2+ isosurfaces of the Yamada et al. cryo-EM reconstructions (i and j) (7), using the “Fit in Map” program in Chimera (33) to align the filaments to Electron Microscopy Data Bank (EMDB) files EMD-0729 and EMD-0728; note the excellent correspondence between data determined by the two methods. Please note that the docking in (a)–(h) and “Fit in Map” alignment to cryo-EM reconstructions in (i) and (j) did not involve any flexible fitting routines or other manipulation of respective coordinates, i.e., only “raw” PIPER/ClusPro output was evaluated. The pointed end of actin is facing up in (a)–(j); arrows mark the tropomyosin overlapping domain; arrowheads mark the troponin core domain electron density. (k–p) Transverse sections through C-state and B-state poses displayed above were made through residue Lys326 of successive actin subunits (blue), showing electrostatic contacts formed by these basic residues on actin and acidic residues along tropomyosin (red), including Glu23, Glu62, Glu100, Glu138, Glu177, and Asp258. Here, sectioned B- and C-state poses were superposed for comparison. Note that at the level of tropomyosin residues 23 and 258, which flank the tropomyosin overlapping domain, the helical chains of B-state and C-state coiled coils either lie directly over each other (at residue 23) or diverge from each other marginally (e.g., at residue 258). In contrast, at the level of residues 62, 100, 138, and 177, the outermost chains diverge from each other by a 50–60° twist like the hands on a clock moving from ∼10 o’clock to noon, whereas those positioned closest to Lys326 still coincide. A picture emerges from these results involving tropomyosin overlapping domains restrained over F-actin under these conditions, whereas the tropomyosin midpiece pivots between B- and C-states. (k–p) Sections are viewed from filament barbed to pointed ends. To see this figure in color, go online.