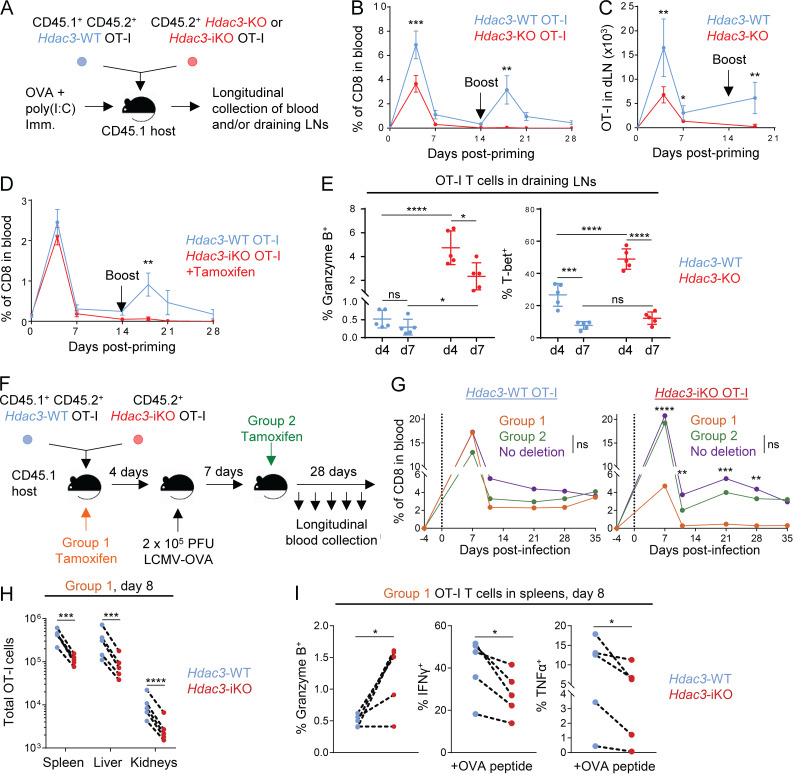
Figure 2.
HDAC3 is required during T cell activation for persistence of the CD8 T cell response. (A) Experimental scheme for longitudinal tracking of OT-I T cell persistence following transfer and OVA immunization. 5 × 105 cells of a 1:1 mix of congenically distinct Hdac3-KO and Hdac3-WT OT-I T cells were transferred into CD45.1+ TCR-polyclonal recipients. Alternatively, 5 × 105 cells of a 1:1 mix of congenically distinct Hdac3-iKO and Hdac3-WT OT-I T cells were transferred, and 2 mg of tamoxifen was administered daily for 3 d to induce Cre-mediated inactivation of Hdac3. Mice were immunized subcutaneously with OVA + poly(I:C) in PBS. A secondary boost of OVA + poly(I:C) was administered on day 14. (B and C) Dynamics of Hdac3-KO and Hdac3-WT OT-I T cell responses were tracked longitudinally in peripheral blood (B) and inguinal LNs (C) draining the immunization site following immunization as described in A. Data are representative of two independent experiments with four or five recipient mice each. (D) Dynamics of Hdac3-iKO and Hdac3-WT OT-I T cell responses were tracked longitudinally in peripheral blood following immunization as in A. Data are representative of two independent experiments with five recipient mice each. (E) Quantification of flow-cytometric analysis of Granzyme B+ and T-bet+ OT-I T cells on days 4 and 7 following transfer and immunization as in A. Data are representative of two independent experiments with five recipient mice each. (F) Experimental scheme for longitudinal tracking of OT-I T cell persistence following transfer in an acute viral infection model. 5 × 103 cells of a 1:1 mix of congenically distinct Hdac3-iKO and Hdac3-WT OT-I T cells were transferred into CD45.1+ TCR-polyclonal recipients. Mice were then infected intraperitoneally with 2 × 105 PFU of LCMV Armstrong expressing the OVA SIINFEKL epitope (LCMV-OVA), and Hdac3 deletion was induced at the indicated time points by intraperitoneal injection of tamoxifen on 3 consecutive days. (G) Donor OT-I responses in peripheral blood were monitored longitudinally for the indicated treatment groups (Group 1, n = 4; Group 2, n = 4; No deletion, n = 3) after treatment as in F. Data are representative of two independent experiments with three to five recipient mice per treatment group. (H and I) Analysis of total numbers (H) and markers of effector phenotype (I) of cotransferred Hdac3-iKO (Hdac3-inducible knockout) and Hdac3-WT OT-I T cells by flow cytometry 8 d after infection with LCMV-OVA. Data are representative of two independent experiments with five recipient mice each, treated as in F. Data from cotransferred donor cells of each genotype within the same recipient mouse were analyzed as pairs. Means ± SEM (B–D), means ± SD (E), or means (G) are indicated. P values were calculated by two-way ANOVA (B–E and G) or two-tailed ratio-paired t test (H and I). Error bars for G were omitted for clarity of presentation; statistical analysis for this experiment was also performed on the ratios of transferred Hdac3-KO to Hdac3-WT OT-I T cells and is shown in Fig. S5 B. *, P < 0.05, **, P < 0.01, ***, P < 0.001, ****, P < 0.0001; ns, not significant.