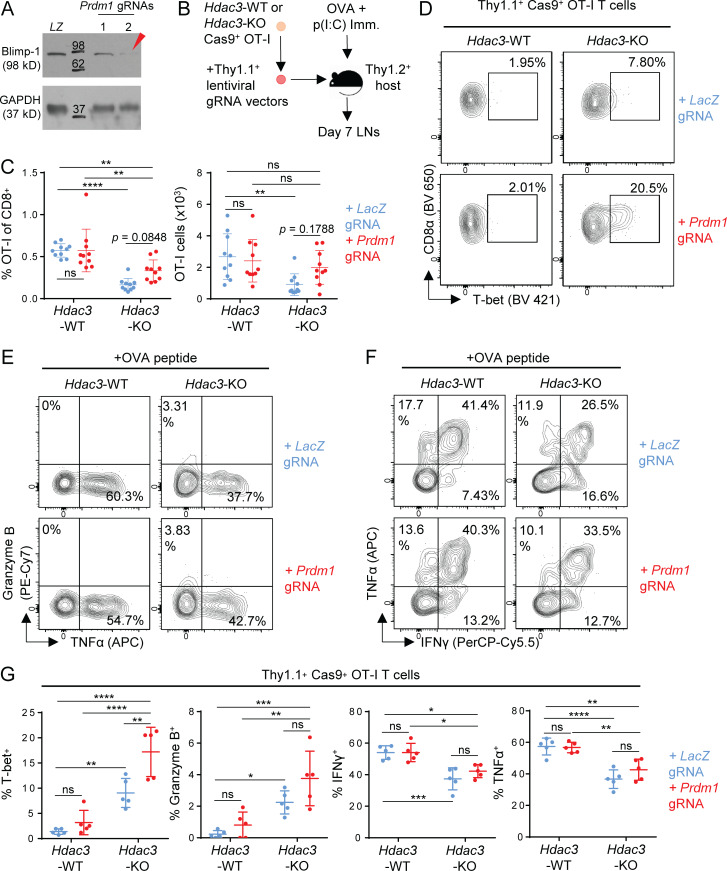
Figure 7.
Inactivation of Prdm1 increases the frequency of T-bet+Hdac3-KO CD8 T cells. (A) Validation of lentiviral gRNA vectors targeting Prdm1 in Cas9+ OT-I T cells. Whole cell lysates from 2 × 105 magnetically purified transduced T cells were loaded per lane for immunoblot analysis. LZ, LacZ-targeting gRNA vector (negative control). Molecular weights (kD) are indicated on immunoblot images. The red arrow indicates the gRNA used in subsequent experiments. (B) Experimental scheme for phenotype analysis of Prdm1-Hdac3-double KO CD8 T cells following in vivo activation. Hdac3-KO and Hdac3-WT Cas9+ OT-I T cells were transduced with lentiviral vectors expressing Prdm1 or LacZ targeting gRNA sequences, magnetically purified based on Thy1.1 expression, and adoptively transferred into Thy1.2+ C57BL/6 recipients. Mice were immunized subcutaneously with OVA and poly(I:C) in PBS. (C) Persistence of Prdm1-Hdac3-double KO CD8 T cells on day 7 after in vivo activation as in B, measured in terms of percentage of transferred OT-I T cells within the total CD8 T cell population (left) and total numbers of OT-I T cells (right) in LNs draining the immunization site. Data are pooled from two independent experiments with five mice per group. (D–G) Flow-cytometric analysis of T-bet (D), Granzyme B (E), and effector cytokine (F) expression in transferred OT-I T cells in inguinal LNs 7 d after OVA immunization as in B. Quantification of flow-cytometric data are shown in G. Data are representative of two independent experiments with five mice per group. Means ± SD are indicated (C and G). P values were calculated by two-way ANOVA (C and G). *, P < 0.05, **, P < 0.01, ***, P < 0.001, ****, P < 0.0001.