FIGURE 1.
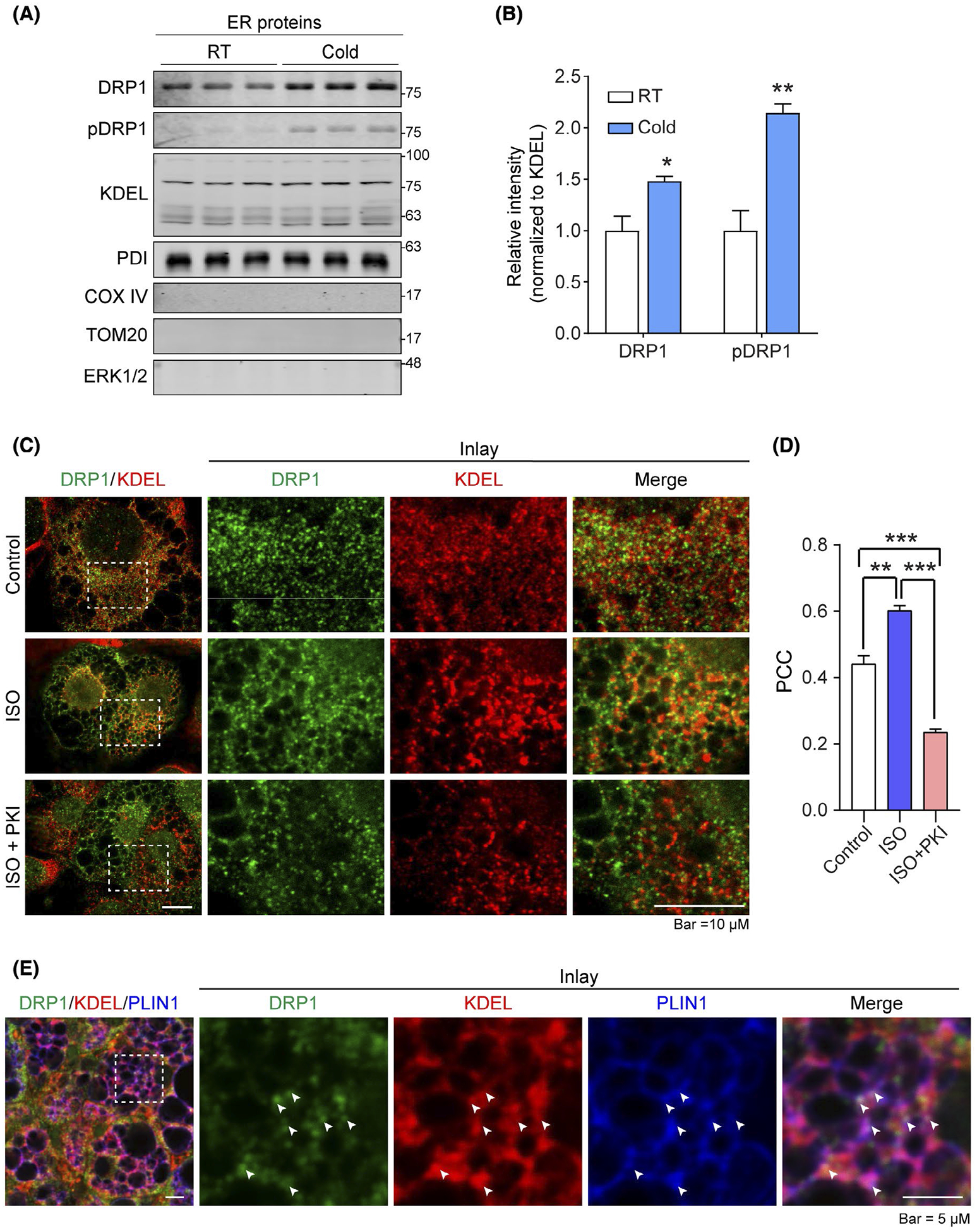
DRP1 translocates onto ER and ER-LD interface in adipose tissue upon β-adrenergic stimulation. A, Western blotting (WB) analysis of DRP1 and pDRP1 (at Ser600 in human, Ser616 in mouse) protein levels in endoplasmic reticulum (ER) fraction isolated from the brown adipose tissue (BAT) of cold exposed or control mice housed at room temperature (RT). KDEL and PDI (ER marker proteins) were used as loading control for ER fraction proteins. COX IV and TOM20 (mitochondrial proteins) were used to verify no mitochondrial contamination in the samples, ERK1/2 was used to verify no cytoplasmic protein contamination (n = 3 per group for each experiment, representative of three experiments). B, Quantification of band intensity for DRP1 and pDPR1 in (A). The data were normalized to the intensity of KDEL (n = 3, data are represented as mean ± SEM, Student’s t test, *P < .05, **P < .01). C, Co-immunofluorescence (IF) staining with anti-DRP1 and anti-KDEL antibodies in differentiated 3T3–L1 cells. The cells were treated with isoproterenol (ISO) at the presence or absence of PKA inhibitor PKI (Representative of 6 fields from one experiment, which was performed in triplicate). D, Pearson’s correlation coefficient (PCC) for colocalization of DRP1 and KDEL in (C) (n = 6, data are represented as mean ± SEM, One-way analysis of variance (ANOVA), **P < .01, ***P < .001). E, Co-IF staining with anti-DRP1, anti-KDEL, and anti-Perilipin-1 (PLIN1) (lipid droplet marker protein) antibodies in the subcutaneous white adipose tissue (sWAT) from cold exposed mice. Arrows indicate the merged signals (puncta with white color) of three channels of red, green, and blue (Representative of 6 fields, experiments were repeated for 3 times)
