The title compound represents the first example of a diphosphane-bridged heterobimetallic Fe—Pt dimetallacyclopentenone complex resulting from a bimetallic activation of metal-coordinated carbonyl ligand with an internal alkyne, namely 1,4-bis(p-tolylthio)but-2-yne. The bridging μ2-C(= O)C(CH2SC6H4Me-4)=CCH2SC6H4Me-4 unit (stemming from a carbon–carbon coupling reaction between CO and the triple bond of the alkyne dithioether) forms a five-membered dimetallacyclopentenone ring, in which the C=C bond is π-coordinated to the Fe center.
Keywords: crystal structure, internal alkyne, iron, platinum, heterobimetallic, metal–metal bond, dimetallcyclopentenone, bis(diphenylphosphino)methane, thioether, hydrogen bonding
Abstract
The title compound, [FePt(C19H18OS2)(C18H15P)(C25H22P2)(CO)2], 1, [(OC)2Fe(μ-dppm)(μ-C(=O)C(CH2SC6H4Me-4)=CCH2SC6H4Me-4)Pt(PPh3)], represents the first example of a diphosphane-bridged heterobimetallic Fe—Pt dimetallacyclopentenone complex resulting from a bimetallic activation of metal-coordinated carbonyl ligand with an internal alkyne, namely 1,4-bis(p-tolylthio)but-2-yne. The bridging μ2-C(=O)C(CH2SC6H4Me-4)=CCH2SC6H4Me-4 unit (stemming from a carbon–carbon coupling reaction between CO and the triple bond of the alkyne dithioether) forms a five-membered dimetallacyclopentenone ring, in which the C=C bond is π-coordinated to the Fe center. The latter is connected to the Pt center through a short metal–metal bond of 2.5697 (6) Å.
Chemical context
Acetylenic dithioether ligands of type RSCH2C≡CCH2SR (R = aryl, alkyl) have in recent years not only attracted attention as reactive building blocks for further organic transformations (Pourcelot & Cadiot, 1966 ▸; Everhardus & Brandsma; 1978 ▸; Levanova et al., 2015 ▸) but also as promising ligands for coordination chemistry because of their dytopic character, allowing both coordination to soft metal centers through dative M←S bonding and π-bonding via the acetylenic triple bond. In this context, we have explored in a series of several papers the coordination of this ligand family to CuX salts in a self-assembly process to discrete molecular compounds, mono- and bidimensional coordination polymers and three-dimensional MOFs. For example, treatment of CuI with PhSCH2C≡CCH2SPh afforded a three-dimensional network incorporating Cu6(μ3-I) hexagonal prisms as connection nodes (Knorr et al., 2009 ▸; Bai et al., 2018 ▸). In contrast, reaction of BzSCH2C≡CCH2SBz (Bz = benzyl) with both CuI and CuBr provided simple isostructural dinuclear zero-dimensional complexes [{Cu(μ2-X)2Cu}(μ-BzSCH2C≡CCH2SBz)2] (X = I, Br). A far more original material resulted from coordination to CuCl, yielding a luminescent 2D material [{Cu2(μ2-Cl)(μ3-Cl)}(μ-BzSCH2C≡CCH2SBz)]n, in which the layers are assembled both by dative Cu—S thioether bonds and organometallic Cu-π–acetylenic interactions via the triple bond of the ligand. Furthermore, the CuI centers are interconnected through μ2- and μ3-bound chloro ligands. Treatment of CuI with the isomeric p-TolSCH2C≡CCH2STol-p (Tol = C6H4-p-Me) ligand led to the formation of a 2D network [{Cu4(μ3-I)4}(μ-TolSCH2C≡CCH2STol)2]n with closed cubane-type clusters as SBUs (Secondary Building Units), whilst with CuBr the 1D [{Cu(μ2-Br)2Cu}(μ-TolSCH2C≡CCH2STol)2]n coordination polymer was generated (Aly et al., 2014 ▸; Bonnot et al., 2015 ▸). An alternative approach to combining a metallic scaffold with RSCH2C≡CCH2SR-type ligands has been developed by Went and coworkers, who post-functionalized dicobaltatetrahedrane complexes [Co2(μ-HOCH2C≡CCH2OH)(CO)6] in the presence of HBF4·OEt2 and various thiols RSH to obtain [Co2(μ-RSCH2C≡CCH2SR)(CO)6] and [Co2(μ-RSCH2C≡CCH2SR)(μ-dppm)(CO)4] [dppm = bis(diphenylphosphino)methane], respectively. Similar treatment of [Mo2(μ-HOCH2C≡CCH2OH)(CO)4Cp2] with EtSH yielded [Mo2(μ-EtSCH2C≡CCH2SEt)(CO)4Cp2]. These former Co–Co thioether complexes were then employed as metalloligands to coordinate further metal fragments such as [Cu(MeCN)4]PF6, AgBF4 and [Mo(CO)4(norbornadiene)] (Bennett, et al., 1992 ▸; Gelling et al., 1993 ▸). Related dicationic salts such as [(Co2(CO)6)2-μ,η2,η2-(Me2S—CH2C≡CCH2SMe2)][BF4]2 have also been described (Amouri et al., 2000 ▸). We and Shaw’s group have demonstrated that upon treatment of the μ-carbonyl complex [(OC)3Fe(μ-dppm)(μ-CO)Pt(PPh3)] with ArC≡CH (Ar = Ph, p-Tol, 2,4,5-trimethylphenyl, p-C6H4F, 2,4-C6H3F2, p-C6H4CF3), dimetallacyclopentone complexes are formed, stemming from carbon–carbon coupling reactions between CO and the terminal alkyne (Fontaine et al., 1988 ▸; Jourdain et al., 2013 ▸; Knorr & Jourdain, 2017 ▸; Brieger et al., 2019 ▸). The first step involves the formation of a kinetic isomer [(OC)2Fe(μ-dppm){μ-C(=O)C(H)=C(Ar)}Pt(PPh3)], which then evolves to the thermodynamic one [(OC)2Fe(μ-dppm){μ-C(=O)C(Ar)=C(H)}Pt(PPh3)]. We were now intrigued as to whether this route may be extended to internal alkynes RC≡CR, which are in general less reactive than terminal ones. We therefore probed the possibility of coupling [(OC)3Fe(μ-dppm)(μ-CO)Pt(PPh3)] with p-TolSCH2C≡CCH2STol-p in hot toluene as solvent and succeeded in isolating the targeted dimetallacyclopentone [(OC)2Fe(μ-dppm)(μ-C(=O)C(4-MeC6H4SCH2)=CCH2SC6H4Me-4)Pt(PPh3)] (1) as a stable crystalline product according to the reaction scheme shown in Fig. 1 ▸. With this title compound 1 in hand, we now have the possibility of coordinating other metal fragments in upcoming studies, for example [Mo(CO)4(norbornadiene)] or ReBr(CO)5 in a chelating manner using the two adjacent thioether arms or of constructing coordination networks incorporating complex 1 as an organometallic building block by coordination of CuX or AgI salts on the S-donor sites (see above).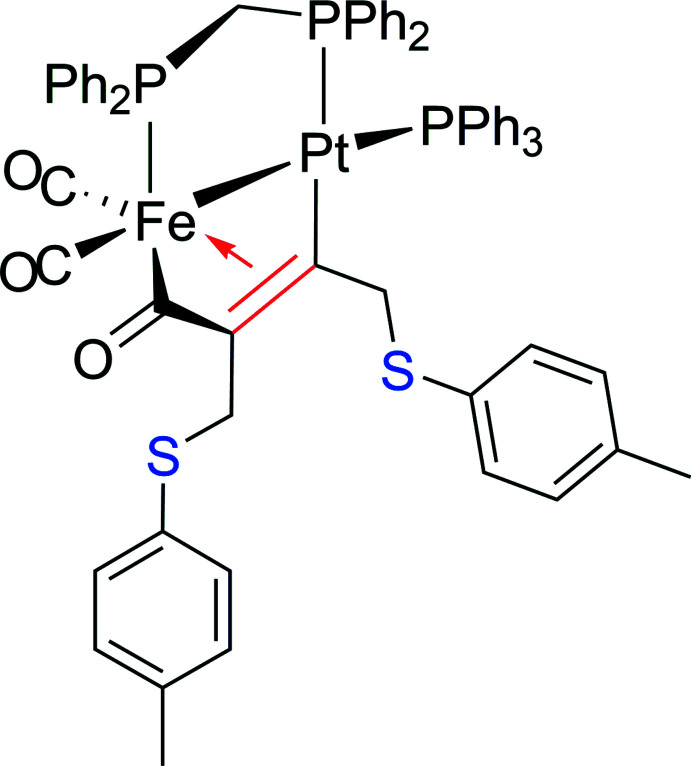
Figure 1.
The reaction scheme for the synthesis of 1.
Structural commentary
The heterobimetallic compound 1 crystallizes in the monoclinic crystal system, space group P21/c. The molecular structure is depicted in Fig. 2 ▸ and selected bond lengths and angles are given in Table 1 ▸.
Figure 2.
The molecular structure of the title complex 1, with atom labeling. Displacement ellipsoids are drawn at the 30% probability level.
Table 1. Selected geometric parameters (Å, °).
| Pt1—Fe1 | 2.5697 (6) | Fe1—C2 | 2.119 (4) |
| Pt1—P2 | 2.2850 (10) | Fe1—C3 | 1.932 (5) |
| Pt1—P3 | 2.2714 (12) | Fe1—C20 | 1.777 (5) |
| Pt1—C1 | 2.045 (4) | Fe1—C21 | 1.789 (5) |
| Fe1—P1 | 2.1966 (12) | O3—C3 | 1.216 (5) |
| Fe1—C1 | 2.162 (4) | C1—C2 | 1.407 (6) |
| P2—Pt1—Fe1 | 97.26 (3) | C1—Fe1—C2 | 38.35 (16) |
| P3—Pt1—Fe1 | 161.46 (3) | C20—Fe1—P1 | 95.66 (14) |
| P3—Pt1—P2 | 100.53 (4) | C21—Fe1—P1 | 102.63 (13) |
| C1—Pt1—Fe1 | 54.44 (12) | C3—Fe1—P1 | 88.88 (13) |
| C1—Pt1—P2 | 151.36 (12) | C1—Fe1—P1 | 141.85 (12) |
| C1—Pt1—P3 | 107.33 (12) | C2—Fe1—P1 | 130.91 (13) |
| Pt1—C1—Fe1 | 75.25 (13) | C20—Fe1—Pt1 | 168.78 (14) |
| C20—Fe1—C3 | 101.5 (2) | C21—Fe1—Pt1 | 87.76 (14) |
| C20—Fe1—C21 | 96.5 (2) | C2—Fe1—Pt1 | 73.72 (12) |
| C21—Fe1—C3 | 157.6 (2) | C1—Fe1—Pt1 | 50.30 (11) |
| C20—Fe1—C1 | 119.19 (18) | C3—Fe1—Pt1 | 72.18 (13) |
| C21—Fe1—C1 | 89.16 (18) | P1—Fe1—Pt1 | 93.50 (4) |
| C3—Fe1—C1 | 70.43 (18) | C2—C1—Pt1 | 109.1 (3) |
| C20—Fe1—C2 | 95.33 (18) | C2—C1—Fe1 | 69.2 (2) |
The Fe—Pt bond [2.5697 (6) Å] is spanned by a dppm ligand and bridged by the C(=O)C(R)=C(R) (R = 4-MeC6H4SCH2) unit resulting from the carbon–carbon coupling reaction between CO and the alkyne. This value, which is less than 2.6 Å, is in the usual range for FePt(dppm)–dimetallacyclopentenone complexes. Note that extreme Fe—Pt distances are reported for the μ-carbene [(OC)3Fe{μ-C(Et)OSi(OMe)3}(μ-dppm)Pt(PPh3)] [d(Fe—Pt) = 2.5062 (9) Å; YOTCIT; Braunstein et al., 1995 ▸] and [Fe(η5-C5H4S)2Pt(PPh3)] [d(Fe—Pt = 2.935 (2) Å; FENCUW; Akabori et al., 1987 ▸]. Coupling of an internal alkyne does not affect the structural features of the [FeC(=O)C(R)=C(R)Pt] motif significantly with respect to carbon–carbon coupling with a terminal alkyne. The relevant bond lengths and angles are very similar to those of other Fe—Pt structures published by Fontaine et al. (1988 ▸) and our group (see above). The presence of a bulky substituent on the C1 atom bound to platinum implies a significant reduction of the P3—Pt—P2 angle [100.53 (4)°] concomitant with an increasing value of the angle P3—Pt—C1 of 107.33 (12)°. In related compounds described previously in the literature, these P3—Pt—P2 angles usually lie in the range 103.93 (8) to 106.63 (3)°, as exemplified by [(OC)2Fe(μ-dppm){μ-C(=O)C{(CH2)3CCH}=C(H)}Pt(PPh3)] (REDNEU) and [(OC)2Fe(μ-dppm){μ-C(=O)C(p-C6H4CF3)=C(H)}Pt(PPh3)] (PIXLAL), and 98.8 (3) to 104.95 (10)° for P3—Pt—C1 in [(OC)2Fe(μ-dppm){μ-C(=O)C(H)=C(H)}Pt(PPh3)] (FEYBAM) and [(OC)2Fe(μ-dppm){μ-C(=O)C(o,p-C6H3F2)=C(H)}Pt(PPh3)] (PIXKUE) (Fontaine et al., 1988 ▸; Jourdain et al., 2006 ▸, 2013 ▸). The crystal structure of the dithioether p-TolSCH2C≡CCH2STol-p (MULHUZ) was reported by Aly et al. (2014 ▸). After complexation and a coupling reaction with a CO ligand, the C1—C2 bond is considerably longer [1.407 (6) vs 1.266 (5) Å] as a result of the conversion to an olefinic moiety, σ-bound to Pt and η2-coordinated to Fe. The alkyne bending angles are disparate [C1—C2—C4 = 126.2 (4), C2—C1—C12 = 119.6 (4)°] as well as the C1—C12 and C2—C4 distances [d(C1—C12) = 1.483 (6), d(C2—C4) = 1.511 (5) Å]. Compared to 1,4-bis(p-tolylthio)but-2-yne, the C—S bonds are also considerably elongated [d(C4—S1 = 1.830 (4), d(C12—S2) = 1.808 (4), d(C5—S1) = 1.782 (5), d(C13—S2) = 1.771 (4) vs 1.685 (2) and 1.714 (2) Å] but they fit well with those encountered in the dimetallatetrahedrane [Co2{μ-C2(CH2SMe)2Mo(CO)4}(μ-dppm)(CO)4] [d(C—S = 1.827 (4), 1.833 (4),1.790 (5) and 1.819 (5) Å; JIHMUI10; Gelling et al., 1993 ▸].
Supramolecular features
In the crystal, the individual molecules are linked by weak intermolecular interactions; for example a contact between O3′′⋯H39 [d = 2.49 Å and C3′′—O3′′⋯H39 = 138°; symmetry code: (′′) −x + 1, −y + 1, −z + 1] occurs (Fig. 3 ▸, Table 2 ▸. A second, yet still weaker intermolecular interaction of 2.67 Å is observed between the O1′⋯H15 [symmetry code: (′) x, –y +  , z −
, z −  ] atoms of two adjacent molecules. In addition there is an intramolecular contact between O3⋯H34A (d = 2.62 Å and C3—O3⋯H34A = 125°). Furthermore, there are also several loose intermolecular C—H⋯π interactions present; for example a contact between C43—H43 and the midpoint of the C13=C14 double bond [d(H43⋯midpoint) = 2.73 Å and C—H⋯midpoint = 157°] of a tolyl ring attached to S2, as well as between C62—H62 and the C23—C24—C25 atoms of a phenyl ring [d(H62⋯centroid) = 2.64 Å and C—H⋯centroid = 148°] attached at P1. However, since all hydrogen atoms were not refined freely, a more accurate discussion of the bond lengths and angle is not appropriate.
] atoms of two adjacent molecules. In addition there is an intramolecular contact between O3⋯H34A (d = 2.62 Å and C3—O3⋯H34A = 125°). Furthermore, there are also several loose intermolecular C—H⋯π interactions present; for example a contact between C43—H43 and the midpoint of the C13=C14 double bond [d(H43⋯midpoint) = 2.73 Å and C—H⋯midpoint = 157°] of a tolyl ring attached to S2, as well as between C62—H62 and the C23—C24—C25 atoms of a phenyl ring [d(H62⋯centroid) = 2.64 Å and C—H⋯centroid = 148°] attached at P1. However, since all hydrogen atoms were not refined freely, a more accurate discussion of the bond lengths and angle is not appropriate.
Figure 3.
A partial view along the a axis of the crystal packing of the title compound. The hydrogen bonds (Table 2 ▸) are shown as dashed lines.
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C15—H15⋯O1i | 0.93 | 2.67 | 3.316 (6) | 128 |
| C34—H34A⋯O3 | 0.97 | 2.62 | 3.271 (5) | 125 |
| C39—H39⋯O3ii | 0.93 | 2.49 | 3.239 (6) | 138 |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Database survey
Other examples of crystallographically characterized dimetallacyclopentenone complexes are Fe2Cp2(CO)(μ-CO){μ-CH=C(Ph)C(=O)} (DAHTAJ; Boni et al., 2011 ▸), Fe2Cp*2(CO)(μ-CO){μ-C(C≡CH)=CHC(=O)] (JUZHIV; Akita et al., 1993 ▸), Fe2(CO)5(μ-dppm){μ-C(=O)CH=CH} (GACWIQ10; Knox et al., 1995 ▸), Fe2(CO)5(μ-dppm){μ-C(=O)C(Ph)=CH} (PIHMOI; Hitchcock et al., 1993 ▸), Fe2Cp2(CO)(μ-CO){μ-C(COR)=C(Me)C(=O)} (R = Ph, Bu) (SIZNUK, SIZPAS; Wong et al., 1991 ▸), Fe2{(η-C5H4)2SiMe2}(CO)2(μ-CO){μ-C(Ph)=C(H)C(=O)} (ZUZGIK; McKee et al., 1994 ▸), Ru2(CO)4(μ-dppm)2{μ-C(=O)C(CO2Me)=C(CO2Me)} (JITZAN; Johnson & Gladfelter, 1991 ▸), Ru2(CO)4(μ-dppm)2{μ-CH=CHC(=O)} (LIFYUU; Mirza et al., 1994 ▸), Ru2(η-C5HMe4)2(CO)(μ-CO){μ-C(=O)C(R)=C(R)} (R = Et, Me) (NEMVOS, NEMVUY; Horiuchi et al., 2012 ▸), Rh2Cp2(CO)4{μ-C(CF3)=C(CF3)C(=O)} (TFPNRH; Dickson et al., 1981 ▸), Re2Cp*2(CO)2{μ-CH=C{C(=CH2)CH3}C(=O)} (WEZKIV; Casey et al., 1994 ▸). A rare example of a heterodinuclear combination is CpFe{μ-C(=O)C(CMe2OH)=CH}(μ-CO)Ru(CO)Cp* (FEHGOP; Dennett et al., 2005 ▸). We are also aware of OsRu(CO)8{μ-HC=CHC(=O)} (Kiel et al., 2000 ▸), but for the latter compound no structural data are available.
Synthesis and crystallization
[(OC)3Fe(μ-CO)(μ-dppm)Pt(PPh3)] (200 mg, 0.2 mmol) was treated with an excess of 1,4-bis(p-tolylthio)but-2-yne (100 mg, 0.4 mmol) in toluene (5 mL). The solution was stirred at 363 K for 6h. The reaction mixture was filtered, and all volatiles removed under reduced pressure. The brown residue was redissolved in a minimum of toluene. Orange–yellow crystals were isolated by layering with heptane (152 mg, 76% yield).
Calculated for C64H55FeO3P3PtS2 (1279.18 g mol−1): C, 60.05; H, 4.36. Found: C, 59.80; H, 4.21. 1H NMR: δ 2.21 (s, 3H, CH3), 2.28 (s, 3H, CH3), 3.67 (br, 2H, CH2), 3.97(br, 2H, CH2), 4.53 (br, 2H, PCH2P, 2 J PtH = 41), 6.45–7.85 (m, 43H, Ph). 31P{1H} NMR: δ 6.8 (d, Pdppm Pt, 2 J PP = 57, 2 + 3 J PP = 5, 1 J PtP = 2543), 32.7 (d, PPPh3 Pt, 3 J PP = 32, 2 + 3 J PP = 5, 1 J PtP = 3506), 63.4 (dd, Pdppm Fe, 2 J PP = 57, 3 J PP = 32, 1 J PtP = 135). IR(toluene): 1966, 1918s ν(CO), 1696m ν(C=O).
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 3 ▸. All of the hydrogen atoms were placed in geometrically calculated positions and each was assigned a fixed isotropic displacement parameter based on a riding model: C—H = 0.93–0.97 Å with U iso(H) = 1.5U eq(C-methyl) and 1.2U eq(C) for other H atoms.
Table 3. Experimental details.
| Crystal data | |
| Chemical formula | [FePt(C19H18OS2)(C18H15P)(C25H22P2)(CO)2] |
| M r | 1280.05 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 293 |
| a, b, c (Å) | 12.0071 (6), 36.1737 (15), 13.6980 (6) |
| β (°) | 111.970 (5) |
| V (Å3) | 5517.5 (5) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 3.01 |
| Crystal size (mm) | 0.23 × 0.15 × 0.05 |
| Data collection | |
| Diffractometer | Agilent Technologies Xcalibur, Sapphire3 |
| Absorption correction | Multi-scan (CrysAlis PRO; Agilent, 2014 ▸) |
| T min, T max | 0.837, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 47863, 10566, 8245 |
| R int | 0.071 |
| (sin θ/λ)max (Å−1) | 0.611 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.037, 0.076, 1.03 |
| No. of reflections | 10566 |
| No. of parameters | 669 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 1.12, −0.64 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989020007859/pk2630sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989020007859/pk2630Isup3.hkl
CCDC reference: 1996804
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
Crystal data
| [FePt(C19H18OS2)(C18H15P)(C25H22P2)(CO)2] | F(000) = 2576 |
| Mr = 1280.05 | Dx = 1.541 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 12.0071 (6) Å | Cell parameters from 9511 reflections |
| b = 36.1737 (15) Å | θ = 2.7–28.7° |
| c = 13.6980 (6) Å | µ = 3.01 mm−1 |
| β = 111.970 (5)° | T = 293 K |
| V = 5517.5 (5) Å3 | Plate, yellow |
| Z = 4 | 0.23 × 0.15 × 0.05 mm |
Data collection
| Agilent Technologies Xcalibur, Sapphire3 diffractometer | 10566 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 8245 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.071 |
| Detector resolution: 16.0560 pixels mm-1 | θmax = 25.8°, θmin = 2.2° |
| ω scans | h = −11→14 |
| Absorption correction: multi-scan (CrysAlisPro; Agilent, 2014) | k = −44→41 |
| Tmin = 0.837, Tmax = 1.000 | l = −16→16 |
| 47863 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: dual |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.037 | H-atom parameters constrained |
| wR(F2) = 0.076 | w = 1/[σ2(Fo2) + (0.0274P)2 + 0.5475P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.03 | (Δ/σ)max = 0.005 |
| 10566 reflections | Δρmax = 1.12 e Å−3 |
| 669 parameters | Δρmin = −0.64 e Å−3 |
| 0 restraints |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Crystal structure determination of compound 1 was accomplished on an Oxford diffraction Xcalibur S Diffractometer. Suitable crystals of 1 were covered with an inert oil (perfluoropolyalkylether and used for X-ray crystal structure determination. Graphite monochromated Mo-Kα radiation (λ = 0.71073?Å) was used. The processing and finalization of the crystal structure was done with the program Olex2 (Dolomanov, 2009). The crystal structures were solved by intrinsic phasing (SHELXT; Sheldrick, 2015a) and refined against F2 with the full-matrix least-squares method (SHELXL; Sheldrick, 2015b). A multi-scan absorption correction using the CrysAlis RED program (Oxford Diffraction, 2010) was employed. The non-hydrogen atoms were refined anisotropically. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Pt1 | 0.33424 (2) | 0.62472 (2) | 0.31155 (2) | 0.01386 (6) | |
| Fe1 | 0.52875 (5) | 0.66228 (2) | 0.35654 (4) | 0.01541 (14) | |
| S1 | 0.51458 (12) | 0.61606 (4) | 0.02018 (9) | 0.0335 (3) | |
| S2 | 0.19884 (11) | 0.69548 (4) | 0.12684 (8) | 0.0242 (3) | |
| P1 | 0.60180 (10) | 0.64353 (3) | 0.52099 (8) | 0.0160 (3) | |
| P2 | 0.36370 (10) | 0.60649 (3) | 0.47912 (8) | 0.0136 (2) | |
| P3 | 0.16428 (10) | 0.59290 (3) | 0.21914 (8) | 0.0166 (3) | |
| O1 | 0.3898 (3) | 0.72834 (9) | 0.3663 (2) | 0.0305 (8) | |
| O2 | 0.7452 (3) | 0.70095 (9) | 0.3644 (3) | 0.0331 (8) | |
| O3 | 0.6059 (3) | 0.58365 (9) | 0.3412 (2) | 0.0226 (7) | |
| C1 | 0.3927 (4) | 0.64918 (12) | 0.2048 (3) | 0.0167 (10) | |
| C2 | 0.5040 (4) | 0.63378 (12) | 0.2148 (3) | 0.0184 (10) | |
| C3 | 0.5576 (4) | 0.61346 (14) | 0.3138 (3) | 0.0199 (11) | |
| C4 | 0.5728 (4) | 0.64158 (13) | 0.1445 (3) | 0.0214 (10) | |
| H4A | 0.656425 | 0.635059 | 0.181663 | 0.026* | |
| H4B | 0.569106 | 0.667854 | 0.129478 | 0.026* | |
| C5 | 0.5750 (4) | 0.57103 (14) | 0.0594 (3) | 0.0284 (12) | |
| C6 | 0.5322 (5) | 0.54791 (15) | 0.1179 (4) | 0.0356 (13) | |
| H6 | 0.469522 | 0.555719 | 0.137208 | 0.043* | |
| C7 | 0.5815 (5) | 0.51369 (15) | 0.1475 (4) | 0.0413 (15) | |
| H7 | 0.552057 | 0.498782 | 0.187748 | 0.050* | |
| C8 | 0.6741 (5) | 0.50038 (15) | 0.1194 (4) | 0.0398 (14) | |
| C9 | 0.7145 (5) | 0.52324 (16) | 0.0597 (4) | 0.0436 (15) | |
| H9 | 0.775600 | 0.514984 | 0.038988 | 0.052* | |
| C10 | 0.6673 (5) | 0.55811 (16) | 0.0295 (4) | 0.0364 (14) | |
| H10 | 0.696839 | 0.572966 | −0.010742 | 0.044* | |
| C11 | 0.7276 (6) | 0.46250 (16) | 0.1544 (5) | 0.065 (2) | |
| H11A | 0.743383 | 0.459346 | 0.227930 | 0.097* | |
| H11B | 0.801292 | 0.460237 | 0.142632 | 0.097* | |
| H11C | 0.672116 | 0.443892 | 0.114692 | 0.097* | |
| C12 | 0.3296 (4) | 0.67416 (13) | 0.1153 (3) | 0.0208 (11) | |
| H12A | 0.305131 | 0.660195 | 0.050210 | 0.025* | |
| H12B | 0.384620 | 0.693322 | 0.112025 | 0.025* | |
| C13 | 0.1237 (4) | 0.71380 (13) | −0.0009 (3) | 0.0213 (11) | |
| C14 | 0.1814 (4) | 0.72895 (13) | −0.0630 (3) | 0.0256 (11) | |
| H14 | 0.264781 | 0.728200 | −0.039538 | 0.031* | |
| C15 | 0.1159 (4) | 0.74513 (14) | −0.1592 (3) | 0.0299 (12) | |
| H15 | 0.156559 | 0.755564 | −0.198448 | 0.036* | |
| C16 | −0.0078 (4) | 0.74615 (13) | −0.1983 (3) | 0.0264 (11) | |
| C17 | −0.0649 (4) | 0.73051 (14) | −0.1363 (3) | 0.0286 (12) | |
| H17 | −0.148331 | 0.730799 | −0.160551 | 0.034* | |
| C18 | −0.0010 (4) | 0.71470 (13) | −0.0406 (3) | 0.0252 (11) | |
| H18 | −0.041978 | 0.704399 | −0.001440 | 0.030* | |
| C19 | −0.0794 (5) | 0.76391 (16) | −0.3034 (4) | 0.0428 (15) | |
| H19A | −0.047412 | 0.787999 | −0.306543 | 0.064* | |
| H19B | −0.161883 | 0.766129 | −0.310870 | 0.064* | |
| H19C | −0.074281 | 0.748835 | −0.359219 | 0.064* | |
| C20 | 0.6604 (4) | 0.68613 (13) | 0.3621 (3) | 0.0230 (11) | |
| C21 | 0.4445 (4) | 0.70239 (13) | 0.3626 (3) | 0.0199 (10) | |
| C22 | 0.7607 (4) | 0.62972 (13) | 0.5705 (3) | 0.0176 (10) | |
| C23 | 0.8454 (4) | 0.65829 (14) | 0.5896 (3) | 0.0246 (11) | |
| H23 | 0.820210 | 0.682797 | 0.581983 | 0.030* | |
| C24 | 0.9660 (4) | 0.65016 (16) | 0.6197 (3) | 0.0326 (13) | |
| H24 | 1.021532 | 0.669251 | 0.632344 | 0.039* | |
| C25 | 1.0048 (4) | 0.61401 (16) | 0.6313 (3) | 0.0324 (14) | |
| H25 | 1.085863 | 0.608652 | 0.649885 | 0.039* | |
| C26 | 0.9219 (4) | 0.58565 (15) | 0.6149 (3) | 0.0315 (13) | |
| H26 | 0.947659 | 0.561191 | 0.624560 | 0.038* | |
| C27 | 0.8012 (4) | 0.59378 (13) | 0.5841 (3) | 0.0234 (11) | |
| H27 | 0.746189 | 0.574587 | 0.572385 | 0.028* | |
| C28 | 0.6025 (4) | 0.67405 (13) | 0.6288 (3) | 0.0198 (10) | |
| C29 | 0.5891 (5) | 0.71186 (14) | 0.6141 (4) | 0.0324 (13) | |
| H29 | 0.581547 | 0.721995 | 0.549531 | 0.039* | |
| C30 | 0.5869 (5) | 0.73480 (15) | 0.6941 (4) | 0.0423 (15) | |
| H30 | 0.577689 | 0.760181 | 0.683252 | 0.051* | |
| C31 | 0.5983 (5) | 0.71993 (16) | 0.7901 (4) | 0.0358 (13) | |
| H31 | 0.595101 | 0.735182 | 0.843640 | 0.043* | |
| C32 | 0.6144 (5) | 0.68300 (15) | 0.8061 (4) | 0.0344 (13) | |
| H32 | 0.623800 | 0.673087 | 0.871411 | 0.041* | |
| C33 | 0.6168 (4) | 0.65998 (13) | 0.7268 (3) | 0.0250 (11) | |
| H33 | 0.628129 | 0.634720 | 0.739143 | 0.030* | |
| C34 | 0.5268 (4) | 0.60132 (12) | 0.5404 (3) | 0.0150 (10) | |
| H34A | 0.552067 | 0.580369 | 0.509554 | 0.018* | |
| H34B | 0.549824 | 0.596640 | 0.615122 | 0.018* | |
| C35 | 0.3097 (4) | 0.56202 (12) | 0.5064 (3) | 0.0160 (10) | |
| C36 | 0.1925 (4) | 0.55998 (13) | 0.5041 (3) | 0.0218 (11) | |
| H36 | 0.144301 | 0.580982 | 0.488889 | 0.026* | |
| C37 | 0.1481 (5) | 0.52697 (14) | 0.5244 (3) | 0.0289 (12) | |
| H37 | 0.069837 | 0.525755 | 0.522178 | 0.035* | |
| C38 | 0.2195 (5) | 0.49550 (14) | 0.5482 (4) | 0.0326 (13) | |
| H38 | 0.189317 | 0.473274 | 0.561975 | 0.039* | |
| C39 | 0.3353 (5) | 0.49742 (13) | 0.5512 (3) | 0.0283 (12) | |
| H39 | 0.383817 | 0.476506 | 0.567991 | 0.034* | |
| C40 | 0.3792 (4) | 0.53025 (13) | 0.5296 (3) | 0.0216 (11) | |
| H40 | 0.457018 | 0.531125 | 0.530449 | 0.026* | |
| C41 | 0.3264 (4) | 0.63622 (12) | 0.5696 (3) | 0.0173 (10) | |
| C42 | 0.2880 (4) | 0.67210 (13) | 0.5430 (3) | 0.0237 (11) | |
| H42 | 0.276531 | 0.680940 | 0.476120 | 0.028* | |
| C43 | 0.2662 (5) | 0.69537 (14) | 0.6159 (4) | 0.0348 (13) | |
| H43 | 0.240436 | 0.719544 | 0.597655 | 0.042* | |
| C44 | 0.2831 (4) | 0.68214 (15) | 0.7138 (4) | 0.0334 (13) | |
| H44 | 0.269937 | 0.697685 | 0.762477 | 0.040* | |
| C45 | 0.3193 (4) | 0.64629 (15) | 0.7419 (3) | 0.0295 (12) | |
| H45 | 0.328276 | 0.637497 | 0.808180 | 0.035* | |
| C46 | 0.3421 (4) | 0.62350 (13) | 0.6706 (3) | 0.0203 (10) | |
| H46 | 0.368134 | 0.599408 | 0.689833 | 0.024* | |
| C47 | 0.1854 (4) | 0.54327 (12) | 0.2099 (3) | 0.0190 (10) | |
| C48 | 0.0931 (4) | 0.52009 (13) | 0.1484 (3) | 0.0258 (11) | |
| H48 | 0.018084 | 0.530064 | 0.109826 | 0.031* | |
| C49 | 0.1112 (5) | 0.48275 (14) | 0.1440 (4) | 0.0328 (13) | |
| H49 | 0.047654 | 0.467587 | 0.104805 | 0.039* | |
| C50 | 0.2232 (5) | 0.46751 (15) | 0.1974 (4) | 0.0367 (14) | |
| H50 | 0.234927 | 0.442181 | 0.194608 | 0.044* | |
| C51 | 0.3172 (5) | 0.49003 (15) | 0.2546 (4) | 0.0359 (13) | |
| H51 | 0.393402 | 0.480116 | 0.288828 | 0.043* | |
| C52 | 0.2979 (4) | 0.52767 (13) | 0.2611 (3) | 0.0227 (11) | |
| H52 | 0.361714 | 0.542713 | 0.300490 | 0.027* | |
| C53 | 0.0484 (4) | 0.60009 (13) | 0.2734 (3) | 0.0186 (10) | |
| C54 | 0.0450 (4) | 0.63486 (13) | 0.3149 (3) | 0.0231 (11) | |
| H54 | 0.102310 | 0.652383 | 0.316344 | 0.028* | |
| C55 | −0.0423 (4) | 0.64388 (14) | 0.3541 (3) | 0.0281 (12) | |
| H55 | −0.043485 | 0.667238 | 0.382068 | 0.034* | |
| C56 | −0.1277 (4) | 0.61781 (14) | 0.3515 (3) | 0.0294 (12) | |
| H56 | −0.185895 | 0.623613 | 0.378580 | 0.035* | |
| C57 | −0.1272 (4) | 0.58350 (14) | 0.3091 (3) | 0.0256 (12) | |
| H57 | −0.186297 | 0.566375 | 0.305649 | 0.031* | |
| C58 | −0.0381 (4) | 0.57431 (14) | 0.2713 (3) | 0.0235 (11) | |
| H58 | −0.036596 | 0.550782 | 0.244489 | 0.028* | |
| C59 | 0.0822 (4) | 0.60429 (12) | 0.0805 (3) | 0.0188 (10) | |
| C60 | −0.0369 (4) | 0.61515 (12) | 0.0426 (3) | 0.0223 (11) | |
| H60 | −0.077921 | 0.616660 | 0.088156 | 0.027* | |
| C61 | −0.0950 (4) | 0.62378 (14) | −0.0626 (3) | 0.0298 (12) | |
| H61 | −0.175186 | 0.630961 | −0.087322 | 0.036* | |
| C62 | −0.0358 (5) | 0.62189 (14) | −0.1314 (3) | 0.0312 (12) | |
| H62 | −0.074977 | 0.628004 | −0.201958 | 0.037* | |
| C63 | 0.0821 (5) | 0.61082 (14) | −0.0938 (3) | 0.0299 (12) | |
| H63 | 0.122595 | 0.609225 | −0.139794 | 0.036* | |
| C64 | 0.1415 (4) | 0.60201 (13) | 0.0108 (3) | 0.0244 (11) | |
| H64 | 0.221358 | 0.594538 | 0.034901 | 0.029* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Pt1 | 0.01128 (9) | 0.01589 (10) | 0.01397 (8) | −0.00082 (8) | 0.00420 (7) | 0.00141 (7) |
| Fe1 | 0.0129 (3) | 0.0159 (4) | 0.0171 (3) | −0.0014 (3) | 0.0053 (3) | 0.0025 (3) |
| S1 | 0.0380 (8) | 0.0434 (9) | 0.0191 (6) | 0.0099 (7) | 0.0109 (6) | 0.0031 (6) |
| S2 | 0.0228 (7) | 0.0292 (7) | 0.0210 (6) | 0.0072 (6) | 0.0087 (5) | 0.0055 (5) |
| P1 | 0.0125 (6) | 0.0177 (7) | 0.0162 (5) | −0.0015 (5) | 0.0035 (5) | 0.0004 (5) |
| P2 | 0.0119 (6) | 0.0134 (6) | 0.0149 (5) | 0.0001 (5) | 0.0043 (5) | 0.0015 (5) |
| P3 | 0.0129 (6) | 0.0200 (7) | 0.0159 (5) | −0.0013 (5) | 0.0043 (5) | −0.0010 (5) |
| O1 | 0.026 (2) | 0.026 (2) | 0.038 (2) | 0.0081 (17) | 0.0105 (17) | −0.0009 (16) |
| O2 | 0.017 (2) | 0.032 (2) | 0.051 (2) | −0.0051 (17) | 0.0128 (17) | 0.0101 (17) |
| O3 | 0.0226 (19) | 0.0190 (19) | 0.0261 (17) | 0.0048 (15) | 0.0091 (15) | 0.0030 (14) |
| C1 | 0.018 (3) | 0.015 (3) | 0.016 (2) | −0.004 (2) | 0.0038 (19) | 0.0017 (18) |
| C2 | 0.021 (3) | 0.017 (3) | 0.018 (2) | −0.003 (2) | 0.009 (2) | −0.0023 (19) |
| C3 | 0.012 (2) | 0.027 (3) | 0.022 (2) | −0.003 (2) | 0.008 (2) | −0.003 (2) |
| C4 | 0.023 (3) | 0.019 (3) | 0.022 (2) | −0.001 (2) | 0.008 (2) | 0.000 (2) |
| C5 | 0.028 (3) | 0.036 (3) | 0.018 (2) | −0.001 (2) | 0.006 (2) | −0.009 (2) |
| C6 | 0.036 (3) | 0.046 (4) | 0.029 (3) | −0.002 (3) | 0.016 (3) | −0.005 (3) |
| C7 | 0.063 (4) | 0.029 (3) | 0.030 (3) | −0.006 (3) | 0.016 (3) | −0.002 (2) |
| C8 | 0.045 (4) | 0.032 (4) | 0.032 (3) | 0.002 (3) | 0.002 (3) | −0.010 (3) |
| C9 | 0.036 (4) | 0.045 (4) | 0.050 (3) | 0.006 (3) | 0.016 (3) | −0.013 (3) |
| C10 | 0.034 (3) | 0.044 (4) | 0.034 (3) | −0.007 (3) | 0.017 (3) | −0.009 (3) |
| C11 | 0.082 (5) | 0.040 (4) | 0.062 (4) | 0.020 (4) | 0.015 (4) | −0.003 (3) |
| C12 | 0.014 (3) | 0.026 (3) | 0.023 (2) | −0.002 (2) | 0.008 (2) | 0.003 (2) |
| C13 | 0.019 (3) | 0.024 (3) | 0.019 (2) | 0.001 (2) | 0.004 (2) | 0.002 (2) |
| C14 | 0.016 (3) | 0.036 (3) | 0.023 (2) | 0.004 (2) | 0.005 (2) | 0.007 (2) |
| C15 | 0.026 (3) | 0.042 (3) | 0.023 (3) | 0.001 (3) | 0.011 (2) | 0.005 (2) |
| C16 | 0.026 (3) | 0.027 (3) | 0.025 (3) | 0.002 (2) | 0.007 (2) | 0.000 (2) |
| C17 | 0.015 (3) | 0.033 (3) | 0.032 (3) | 0.003 (2) | 0.004 (2) | 0.001 (2) |
| C18 | 0.019 (3) | 0.031 (3) | 0.026 (3) | 0.002 (2) | 0.010 (2) | 0.004 (2) |
| C19 | 0.035 (3) | 0.054 (4) | 0.034 (3) | 0.004 (3) | 0.006 (3) | 0.011 (3) |
| C20 | 0.020 (3) | 0.022 (3) | 0.026 (2) | 0.005 (2) | 0.007 (2) | 0.004 (2) |
| C21 | 0.016 (3) | 0.021 (3) | 0.021 (2) | −0.003 (2) | 0.006 (2) | 0.003 (2) |
| C22 | 0.009 (2) | 0.027 (3) | 0.015 (2) | 0.002 (2) | 0.0027 (18) | 0.0000 (19) |
| C23 | 0.020 (3) | 0.033 (3) | 0.020 (2) | −0.001 (2) | 0.006 (2) | 0.000 (2) |
| C24 | 0.017 (3) | 0.056 (4) | 0.022 (3) | −0.014 (3) | 0.006 (2) | −0.002 (3) |
| C25 | 0.010 (3) | 0.066 (4) | 0.021 (2) | 0.002 (3) | 0.005 (2) | −0.006 (3) |
| C26 | 0.020 (3) | 0.040 (3) | 0.030 (3) | 0.011 (3) | 0.005 (2) | −0.011 (2) |
| C27 | 0.021 (3) | 0.028 (3) | 0.019 (2) | 0.005 (2) | 0.005 (2) | −0.003 (2) |
| C28 | 0.013 (2) | 0.019 (3) | 0.023 (2) | 0.000 (2) | 0.002 (2) | −0.004 (2) |
| C29 | 0.035 (3) | 0.024 (3) | 0.026 (3) | −0.001 (2) | −0.003 (2) | −0.006 (2) |
| C30 | 0.041 (4) | 0.025 (3) | 0.046 (3) | 0.006 (3) | 0.001 (3) | −0.011 (3) |
| C31 | 0.027 (3) | 0.042 (4) | 0.038 (3) | 0.000 (3) | 0.012 (2) | −0.021 (3) |
| C32 | 0.032 (3) | 0.043 (4) | 0.031 (3) | −0.011 (3) | 0.016 (2) | −0.010 (3) |
| C33 | 0.030 (3) | 0.018 (3) | 0.028 (3) | −0.001 (2) | 0.011 (2) | −0.003 (2) |
| C34 | 0.015 (2) | 0.016 (3) | 0.012 (2) | 0.002 (2) | 0.0037 (18) | 0.0012 (18) |
| C35 | 0.019 (3) | 0.017 (3) | 0.010 (2) | −0.002 (2) | 0.0045 (19) | 0.0010 (18) |
| C36 | 0.021 (3) | 0.021 (3) | 0.020 (2) | −0.002 (2) | 0.004 (2) | 0.000 (2) |
| C37 | 0.028 (3) | 0.032 (3) | 0.029 (3) | −0.013 (3) | 0.013 (2) | −0.006 (2) |
| C38 | 0.048 (4) | 0.019 (3) | 0.034 (3) | −0.017 (3) | 0.018 (3) | −0.001 (2) |
| C39 | 0.038 (3) | 0.016 (3) | 0.029 (3) | 0.005 (2) | 0.010 (2) | 0.000 (2) |
| C40 | 0.021 (3) | 0.022 (3) | 0.022 (2) | 0.000 (2) | 0.008 (2) | 0.003 (2) |
| C41 | 0.011 (2) | 0.018 (3) | 0.023 (2) | −0.005 (2) | 0.0069 (19) | −0.0025 (19) |
| C42 | 0.027 (3) | 0.021 (3) | 0.027 (2) | 0.000 (2) | 0.015 (2) | −0.001 (2) |
| C43 | 0.039 (3) | 0.020 (3) | 0.052 (3) | 0.003 (3) | 0.025 (3) | −0.006 (3) |
| C44 | 0.026 (3) | 0.044 (4) | 0.036 (3) | −0.009 (3) | 0.017 (2) | −0.022 (3) |
| C45 | 0.025 (3) | 0.044 (4) | 0.021 (2) | −0.003 (3) | 0.010 (2) | −0.004 (2) |
| C46 | 0.015 (2) | 0.022 (3) | 0.023 (2) | −0.004 (2) | 0.0062 (19) | −0.001 (2) |
| C47 | 0.022 (3) | 0.019 (3) | 0.019 (2) | 0.000 (2) | 0.010 (2) | 0.0020 (19) |
| C48 | 0.021 (3) | 0.027 (3) | 0.027 (3) | −0.004 (2) | 0.006 (2) | −0.004 (2) |
| C49 | 0.037 (3) | 0.027 (3) | 0.030 (3) | −0.012 (3) | 0.008 (3) | −0.007 (2) |
| C50 | 0.057 (4) | 0.021 (3) | 0.031 (3) | 0.001 (3) | 0.016 (3) | 0.000 (2) |
| C51 | 0.035 (3) | 0.033 (3) | 0.035 (3) | 0.008 (3) | 0.008 (3) | 0.004 (3) |
| C52 | 0.026 (3) | 0.017 (3) | 0.022 (2) | −0.004 (2) | 0.005 (2) | −0.005 (2) |
| C53 | 0.013 (3) | 0.027 (3) | 0.014 (2) | −0.002 (2) | 0.0023 (19) | 0.0020 (19) |
| C54 | 0.016 (3) | 0.028 (3) | 0.023 (2) | 0.001 (2) | 0.005 (2) | 0.003 (2) |
| C55 | 0.026 (3) | 0.032 (3) | 0.029 (3) | 0.009 (3) | 0.014 (2) | 0.001 (2) |
| C56 | 0.020 (3) | 0.046 (4) | 0.025 (2) | 0.006 (3) | 0.011 (2) | 0.005 (2) |
| C57 | 0.014 (3) | 0.036 (3) | 0.025 (2) | 0.000 (2) | 0.006 (2) | 0.010 (2) |
| C58 | 0.017 (3) | 0.033 (3) | 0.020 (2) | −0.004 (2) | 0.005 (2) | 0.002 (2) |
| C59 | 0.016 (3) | 0.019 (3) | 0.018 (2) | −0.003 (2) | 0.0011 (19) | −0.0021 (19) |
| C60 | 0.018 (3) | 0.027 (3) | 0.022 (2) | 0.006 (2) | 0.008 (2) | 0.007 (2) |
| C61 | 0.020 (3) | 0.036 (3) | 0.024 (2) | 0.007 (2) | −0.002 (2) | −0.001 (2) |
| C62 | 0.034 (3) | 0.037 (3) | 0.015 (2) | 0.001 (3) | 0.001 (2) | 0.001 (2) |
| C63 | 0.036 (3) | 0.040 (3) | 0.017 (2) | 0.001 (3) | 0.014 (2) | −0.001 (2) |
| C64 | 0.016 (3) | 0.034 (3) | 0.022 (2) | 0.001 (2) | 0.005 (2) | −0.002 (2) |
Geometric parameters (Å, º)
| Pt1—Fe1 | 2.5697 (6) | C26—C27 | 1.382 (6) |
| Pt1—P2 | 2.2850 (10) | C27—H27 | 0.9300 |
| Pt1—P3 | 2.2714 (12) | C28—C29 | 1.383 (6) |
| Pt1—C1 | 2.045 (4) | C28—C33 | 1.385 (6) |
| Fe1—P1 | 2.1966 (12) | C29—H29 | 0.9300 |
| Fe1—C1 | 2.162 (4) | C29—C30 | 1.383 (6) |
| Fe1—C2 | 2.119 (4) | C30—H30 | 0.9300 |
| Fe1—C3 | 1.932 (5) | C30—C31 | 1.379 (7) |
| Fe1—C20 | 1.777 (5) | C31—H31 | 0.9300 |
| Fe1—C21 | 1.789 (5) | C31—C32 | 1.356 (7) |
| S1—C4 | 1.830 (4) | C32—H32 | 0.9300 |
| S1—C5 | 1.782 (5) | C32—C33 | 1.378 (6) |
| S2—C12 | 1.808 (4) | C33—H33 | 0.9300 |
| S2—C13 | 1.771 (4) | C34—H34A | 0.9700 |
| P1—C22 | 1.839 (4) | C34—H34B | 0.9700 |
| P1—C28 | 1.841 (4) | C35—C36 | 1.399 (6) |
| P1—C34 | 1.842 (4) | C35—C40 | 1.385 (6) |
| P2—C34 | 1.829 (4) | C36—H36 | 0.9300 |
| P2—C35 | 1.824 (4) | C36—C37 | 1.378 (6) |
| P2—C41 | 1.819 (4) | C37—H37 | 0.9300 |
| P3—C47 | 1.824 (5) | C37—C38 | 1.388 (7) |
| P3—C53 | 1.824 (4) | C38—H38 | 0.9300 |
| P3—C59 | 1.830 (4) | C38—C39 | 1.376 (7) |
| O1—C21 | 1.157 (5) | C39—H39 | 0.9300 |
| O2—C20 | 1.141 (5) | C39—C40 | 1.377 (6) |
| O3—C3 | 1.216 (5) | C40—H40 | 0.9300 |
| C1—C2 | 1.407 (6) | C41—C42 | 1.380 (6) |
| C1—C12 | 1.483 (6) | C41—C46 | 1.402 (6) |
| C2—C3 | 1.464 (6) | C42—H42 | 0.9300 |
| C2—C4 | 1.511 (5) | C42—C43 | 1.404 (6) |
| C4—H4A | 0.9700 | C43—H43 | 0.9300 |
| C4—H4B | 0.9700 | C43—C44 | 1.366 (6) |
| C5—C6 | 1.384 (6) | C44—H44 | 0.9300 |
| C5—C10 | 1.397 (6) | C44—C45 | 1.376 (7) |
| C6—H6 | 0.9300 | C45—H45 | 0.9300 |
| C6—C7 | 1.367 (7) | C45—C46 | 1.381 (6) |
| C7—H7 | 0.9300 | C46—H46 | 0.9300 |
| C7—C8 | 1.392 (7) | C47—C48 | 1.394 (6) |
| C8—C9 | 1.373 (7) | C47—C52 | 1.388 (6) |
| C8—C11 | 1.513 (7) | C48—H48 | 0.9300 |
| C9—H9 | 0.9300 | C48—C49 | 1.373 (7) |
| C9—C10 | 1.381 (7) | C49—H49 | 0.9300 |
| C10—H10 | 0.9300 | C49—C50 | 1.382 (7) |
| C11—H11A | 0.9600 | C50—H50 | 0.9300 |
| C11—H11B | 0.9600 | C50—C51 | 1.374 (7) |
| C11—H11C | 0.9600 | C51—H51 | 0.9300 |
| C12—H12A | 0.9700 | C51—C52 | 1.389 (6) |
| C12—H12B | 0.9700 | C52—H52 | 0.9300 |
| C13—C14 | 1.394 (6) | C53—C54 | 1.387 (6) |
| C13—C18 | 1.390 (6) | C53—C58 | 1.389 (6) |
| C14—H14 | 0.9300 | C54—H54 | 0.9300 |
| C14—C15 | 1.387 (6) | C54—C55 | 1.384 (6) |
| C15—H15 | 0.9300 | C55—H55 | 0.9300 |
| C15—C16 | 1.378 (6) | C55—C56 | 1.383 (7) |
| C16—C17 | 1.395 (6) | C56—H56 | 0.9300 |
| C16—C19 | 1.516 (6) | C56—C57 | 1.371 (7) |
| C17—H17 | 0.9300 | C57—H57 | 0.9300 |
| C17—C18 | 1.373 (6) | C57—C58 | 1.391 (6) |
| C18—H18 | 0.9300 | C58—H58 | 0.9300 |
| C19—H19A | 0.9600 | C59—C60 | 1.383 (6) |
| C19—H19B | 0.9600 | C59—C64 | 1.390 (6) |
| C19—H19C | 0.9600 | C60—H60 | 0.9300 |
| C22—C23 | 1.404 (6) | C60—C61 | 1.382 (6) |
| C22—C27 | 1.376 (6) | C61—H61 | 0.9300 |
| C23—H23 | 0.9300 | C61—C62 | 1.378 (6) |
| C23—C24 | 1.381 (6) | C62—H62 | 0.9300 |
| C24—H24 | 0.9300 | C62—C63 | 1.372 (7) |
| C24—C25 | 1.377 (7) | C63—H63 | 0.9300 |
| C25—H25 | 0.9300 | C63—C64 | 1.379 (6) |
| C25—C26 | 1.388 (7) | C64—H64 | 0.9300 |
| C26—H26 | 0.9300 | ||
| P2—Pt1—Fe1 | 97.26 (3) | C24—C23—C22 | 120.3 (5) |
| P3—Pt1—Fe1 | 161.46 (3) | C24—C23—H23 | 119.9 |
| P3—Pt1—P2 | 100.53 (4) | C23—C24—H24 | 119.7 |
| C1—Pt1—Fe1 | 54.44 (12) | C25—C24—C23 | 120.5 (5) |
| C1—Pt1—P2 | 151.36 (12) | C25—C24—H24 | 119.7 |
| C1—Pt1—P3 | 107.33 (12) | C24—C25—H25 | 120.2 |
| P1—Fe1—Pt1 | 93.50 (4) | C24—C25—C26 | 119.5 (5) |
| C1—Fe1—Pt1 | 50.30 (11) | C26—C25—H25 | 120.2 |
| C1—Fe1—P1 | 141.85 (12) | C25—C26—H26 | 120.0 |
| C2—Fe1—Pt1 | 73.72 (12) | C27—C26—C25 | 119.9 (5) |
| C2—Fe1—P1 | 130.91 (13) | C27—C26—H26 | 120.0 |
| C2—Fe1—C1 | 38.35 (16) | C22—C27—C26 | 121.3 (5) |
| C3—Fe1—Pt1 | 72.18 (13) | C22—C27—H27 | 119.3 |
| C3—Fe1—P1 | 88.88 (13) | C26—C27—H27 | 119.3 |
| C3—Fe1—C1 | 70.43 (18) | C29—C28—P1 | 120.7 (3) |
| C3—Fe1—C2 | 42.03 (17) | C29—C28—C33 | 118.0 (4) |
| C20—Fe1—Pt1 | 168.78 (14) | C33—C28—P1 | 121.2 (4) |
| C20—Fe1—P1 | 95.66 (14) | C28—C29—H29 | 119.5 |
| C20—Fe1—C1 | 119.19 (18) | C28—C29—C30 | 120.9 (5) |
| C20—Fe1—C2 | 95.33 (18) | C30—C29—H29 | 119.5 |
| C20—Fe1—C3 | 101.5 (2) | C29—C30—H30 | 120.1 |
| C20—Fe1—C21 | 96.5 (2) | C31—C30—C29 | 119.8 (5) |
| C21—Fe1—Pt1 | 87.76 (14) | C31—C30—H30 | 120.1 |
| C21—Fe1—P1 | 102.63 (13) | C30—C31—H31 | 120.1 |
| C21—Fe1—C1 | 89.16 (18) | C32—C31—C30 | 119.8 (5) |
| C21—Fe1—C2 | 123.33 (18) | C32—C31—H31 | 120.1 |
| C21—Fe1—C3 | 157.6 (2) | C31—C32—H32 | 119.6 |
| C5—S1—C4 | 102.1 (2) | C31—C32—C33 | 120.7 (5) |
| C13—S2—C12 | 102.2 (2) | C33—C32—H32 | 119.6 |
| C22—P1—Fe1 | 115.03 (13) | C28—C33—H33 | 119.6 |
| C22—P1—C28 | 100.05 (19) | C32—C33—C28 | 120.7 (5) |
| C22—P1—C34 | 102.6 (2) | C32—C33—H33 | 119.6 |
| C28—P1—Fe1 | 121.17 (15) | P1—C34—H34A | 109.6 |
| C28—P1—C34 | 103.71 (19) | P1—C34—H34B | 109.6 |
| C34—P1—Fe1 | 112.02 (13) | P2—C34—P1 | 110.3 (2) |
| C34—P2—Pt1 | 103.14 (13) | P2—C34—H34A | 109.6 |
| C35—P2—Pt1 | 121.79 (13) | P2—C34—H34B | 109.6 |
| C35—P2—C34 | 102.5 (2) | H34A—C34—H34B | 108.1 |
| C41—P2—Pt1 | 121.93 (15) | C36—C35—P2 | 118.3 (3) |
| C41—P2—C34 | 103.99 (19) | C40—C35—P2 | 123.3 (3) |
| C41—P2—C35 | 100.66 (19) | C40—C35—C36 | 118.5 (4) |
| C47—P3—Pt1 | 114.72 (15) | C35—C36—H36 | 119.9 |
| C47—P3—C53 | 108.4 (2) | C37—C36—C35 | 120.2 (5) |
| C47—P3—C59 | 100.6 (2) | C37—C36—H36 | 119.9 |
| C53—P3—Pt1 | 111.32 (15) | C36—C37—H37 | 119.8 |
| C53—P3—C59 | 101.2 (2) | C36—C37—C38 | 120.4 (5) |
| C59—P3—Pt1 | 119.15 (15) | C38—C37—H37 | 119.8 |
| Pt1—C1—Fe1 | 75.25 (13) | C37—C38—H38 | 120.2 |
| C2—C1—Pt1 | 109.1 (3) | C39—C38—C37 | 119.6 (5) |
| C2—C1—Fe1 | 69.2 (2) | C39—C38—H38 | 120.2 |
| C2—C1—C12 | 119.6 (4) | C38—C39—H39 | 120.0 |
| C12—C1—Pt1 | 130.5 (3) | C38—C39—C40 | 120.1 (5) |
| C12—C1—Fe1 | 129.0 (3) | C40—C39—H39 | 120.0 |
| C1—C2—Fe1 | 72.5 (2) | C35—C40—H40 | 119.4 |
| C1—C2—C3 | 111.2 (4) | C39—C40—C35 | 121.2 (4) |
| C1—C2—C4 | 126.2 (4) | C39—C40—H40 | 119.4 |
| C3—C2—Fe1 | 62.1 (2) | C42—C41—P2 | 121.3 (3) |
| C3—C2—C4 | 121.9 (4) | C42—C41—C46 | 118.5 (4) |
| C4—C2—Fe1 | 124.8 (3) | C46—C41—P2 | 120.1 (3) |
| O3—C3—Fe1 | 146.8 (3) | C41—C42—H42 | 119.7 |
| O3—C3—C2 | 136.5 (4) | C41—C42—C43 | 120.6 (4) |
| C2—C3—Fe1 | 75.8 (3) | C43—C42—H42 | 119.7 |
| S1—C4—H4A | 109.0 | C42—C43—H43 | 120.3 |
| S1—C4—H4B | 109.0 | C44—C43—C42 | 119.3 (5) |
| C2—C4—S1 | 112.9 (3) | C44—C43—H43 | 120.3 |
| C2—C4—H4A | 109.0 | C43—C44—H44 | 119.3 |
| C2—C4—H4B | 109.0 | C43—C44—C45 | 121.3 (4) |
| H4A—C4—H4B | 107.8 | C45—C44—H44 | 119.3 |
| C6—C5—S1 | 121.9 (4) | C44—C45—H45 | 120.3 |
| C6—C5—C10 | 118.4 (5) | C44—C45—C46 | 119.4 (4) |
| C10—C5—S1 | 119.7 (4) | C46—C45—H45 | 120.3 |
| C5—C6—H6 | 119.8 | C41—C46—H46 | 119.6 |
| C7—C6—C5 | 120.4 (5) | C45—C46—C41 | 120.8 (4) |
| C7—C6—H6 | 119.8 | C45—C46—H46 | 119.6 |
| C6—C7—H7 | 118.9 | C48—C47—P3 | 122.3 (4) |
| C6—C7—C8 | 122.2 (5) | C52—C47—P3 | 120.0 (3) |
| C8—C7—H7 | 118.9 | C52—C47—C48 | 117.7 (4) |
| C7—C8—C11 | 121.0 (5) | C47—C48—H48 | 119.5 |
| C9—C8—C7 | 116.9 (5) | C49—C48—C47 | 121.0 (5) |
| C9—C8—C11 | 122.1 (5) | C49—C48—H48 | 119.5 |
| C8—C9—H9 | 118.9 | C48—C49—H49 | 119.8 |
| C8—C9—C10 | 122.2 (5) | C48—C49—C50 | 120.5 (5) |
| C10—C9—H9 | 118.9 | C50—C49—H49 | 119.8 |
| C5—C10—H10 | 120.0 | C49—C50—H50 | 120.2 |
| C9—C10—C5 | 119.9 (5) | C51—C50—C49 | 119.6 (5) |
| C9—C10—H10 | 120.0 | C51—C50—H50 | 120.2 |
| C8—C11—H11A | 109.5 | C50—C51—H51 | 120.1 |
| C8—C11—H11B | 109.5 | C50—C51—C52 | 119.8 (5) |
| C8—C11—H11C | 109.5 | C52—C51—H51 | 120.1 |
| H11A—C11—H11B | 109.5 | C47—C52—C51 | 121.3 (4) |
| H11A—C11—H11C | 109.5 | C47—C52—H52 | 119.3 |
| H11B—C11—H11C | 109.5 | C51—C52—H52 | 119.3 |
| S2—C12—H12A | 109.1 | C54—C53—P3 | 116.1 (3) |
| S2—C12—H12B | 109.1 | C54—C53—C58 | 118.7 (4) |
| C1—C12—S2 | 112.3 (3) | C58—C53—P3 | 125.1 (4) |
| C1—C12—H12A | 109.1 | C53—C54—H54 | 119.5 |
| C1—C12—H12B | 109.1 | C55—C54—C53 | 121.0 (5) |
| H12A—C12—H12B | 107.9 | C55—C54—H54 | 119.5 |
| C14—C13—S2 | 124.4 (4) | C54—C55—H55 | 120.3 |
| C18—C13—S2 | 118.1 (3) | C56—C55—C54 | 119.4 (5) |
| C18—C13—C14 | 117.5 (4) | C56—C55—H55 | 120.3 |
| C13—C14—H14 | 119.6 | C55—C56—H56 | 119.8 |
| C15—C14—C13 | 120.7 (4) | C57—C56—C55 | 120.5 (4) |
| C15—C14—H14 | 119.6 | C57—C56—H56 | 119.8 |
| C14—C15—H15 | 119.1 | C56—C57—H57 | 120.0 |
| C16—C15—C14 | 121.7 (4) | C56—C57—C58 | 120.0 (5) |
| C16—C15—H15 | 119.1 | C58—C57—H57 | 120.0 |
| C15—C16—C17 | 117.2 (4) | C53—C58—C57 | 120.4 (5) |
| C15—C16—C19 | 121.7 (4) | C53—C58—H58 | 119.8 |
| C17—C16—C19 | 121.1 (5) | C57—C58—H58 | 119.8 |
| C16—C17—H17 | 119.2 | C60—C59—P3 | 122.2 (3) |
| C18—C17—C16 | 121.6 (5) | C60—C59—C64 | 118.9 (4) |
| C18—C17—H17 | 119.2 | C64—C59—P3 | 118.9 (3) |
| C13—C18—H18 | 119.4 | C59—C60—H60 | 119.9 |
| C17—C18—C13 | 121.2 (4) | C61—C60—C59 | 120.2 (4) |
| C17—C18—H18 | 119.4 | C61—C60—H60 | 119.9 |
| C16—C19—H19A | 109.5 | C60—C61—H61 | 119.6 |
| C16—C19—H19B | 109.5 | C62—C61—C60 | 120.9 (5) |
| C16—C19—H19C | 109.5 | C62—C61—H61 | 119.6 |
| H19A—C19—H19B | 109.5 | C61—C62—H62 | 120.6 |
| H19A—C19—H19C | 109.5 | C63—C62—C61 | 118.8 (4) |
| H19B—C19—H19C | 109.5 | C63—C62—H62 | 120.6 |
| O2—C20—Fe1 | 178.7 (4) | C62—C63—H63 | 119.4 |
| O1—C21—Fe1 | 179.9 (5) | C62—C63—C64 | 121.1 (4) |
| C23—C22—P1 | 116.6 (4) | C64—C63—H63 | 119.4 |
| C27—C22—P1 | 124.9 (4) | C59—C64—H64 | 119.9 |
| C27—C22—C23 | 118.4 (4) | C63—C64—C59 | 120.1 (4) |
| C22—C23—H23 | 119.9 | C63—C64—H64 | 119.9 |
| Pt1—P2—C34—P1 | 52.0 (2) | C22—P1—C28—C29 | 109.4 (4) |
| Pt1—P2—C35—C36 | −87.5 (3) | C22—P1—C28—C33 | −70.4 (4) |
| Pt1—P2—C35—C40 | 92.7 (4) | C22—P1—C34—P2 | −172.0 (2) |
| Pt1—P2—C41—C42 | −5.3 (4) | C22—C23—C24—C25 | 0.1 (6) |
| Pt1—P2—C41—C46 | 177.8 (3) | C23—C22—C27—C26 | −0.6 (6) |
| Pt1—P3—C47—C48 | −174.1 (3) | C23—C24—C25—C26 | −1.6 (7) |
| Pt1—P3—C47—C52 | 2.6 (4) | C24—C25—C26—C27 | 2.0 (7) |
| Pt1—P3—C53—C54 | 34.5 (4) | C25—C26—C27—C22 | −0.9 (7) |
| Pt1—P3—C53—C58 | −148.9 (3) | C27—C22—C23—C24 | 1.0 (6) |
| Pt1—P3—C59—C60 | −122.1 (4) | C28—P1—C22—C23 | −58.9 (3) |
| Pt1—P3—C59—C64 | 57.8 (4) | C28—P1—C22—C27 | 124.9 (4) |
| Pt1—C1—C2—Fe1 | −65.2 (2) | C28—P1—C34—P2 | 84.2 (2) |
| Pt1—C1—C2—C3 | −15.6 (4) | C28—C29—C30—C31 | 0.1 (8) |
| Pt1—C1—C2—C4 | 174.2 (3) | C29—C28—C33—C32 | 1.8 (7) |
| Pt1—C1—C12—S2 | 18.9 (5) | C29—C30—C31—C32 | 1.4 (8) |
| Fe1—P1—C22—C23 | 72.6 (3) | C30—C31—C32—C33 | −1.4 (8) |
| Fe1—P1—C22—C27 | −103.6 (3) | C31—C32—C33—C28 | −0.2 (8) |
| Fe1—P1—C28—C29 | −18.2 (5) | C33—C28—C29—C30 | −1.7 (7) |
| Fe1—P1—C28—C33 | 162.1 (3) | C34—P1—C22—C23 | −165.5 (3) |
| Fe1—P1—C34—P2 | −48.1 (2) | C34—P1—C22—C27 | 18.3 (4) |
| Fe1—C1—C2—C3 | 49.6 (3) | C34—P1—C28—C29 | −144.9 (4) |
| Fe1—C1—C2—C4 | −120.6 (4) | C34—P1—C28—C33 | 35.3 (4) |
| Fe1—C1—C12—S2 | −86.2 (4) | C34—P2—C35—C36 | 158.2 (3) |
| Fe1—C2—C3—O3 | −170.8 (6) | C34—P2—C35—C40 | −21.6 (4) |
| Fe1—C2—C4—S1 | −172.3 (2) | C34—P2—C41—C42 | 110.2 (4) |
| S1—C5—C6—C7 | −179.4 (4) | C34—P2—C41—C46 | −66.6 (4) |
| S1—C5—C10—C9 | 180.0 (4) | C35—P2—C34—P1 | 179.29 (19) |
| S2—C13—C14—C15 | −175.3 (4) | C35—P2—C41—C42 | −143.8 (4) |
| S2—C13—C18—C17 | 176.1 (4) | C35—P2—C41—C46 | 39.3 (4) |
| P1—C22—C23—C24 | −175.4 (3) | C35—C36—C37—C38 | 0.6 (6) |
| P1—C22—C27—C26 | 175.5 (3) | C36—C35—C40—C39 | −0.8 (6) |
| P1—C28—C29—C30 | 178.5 (4) | C36—C37—C38—C39 | −0.1 (7) |
| P1—C28—C33—C32 | −178.5 (4) | C37—C38—C39—C40 | −0.8 (7) |
| P2—C35—C36—C37 | −179.9 (3) | C38—C39—C40—C35 | 1.3 (7) |
| P2—C35—C40—C39 | 179.0 (3) | C40—C35—C36—C37 | −0.1 (6) |
| P2—C41—C42—C43 | −176.3 (4) | C41—P2—C34—P1 | −76.2 (2) |
| P2—C41—C46—C45 | 177.1 (3) | C41—P2—C35—C36 | 51.1 (3) |
| P3—C47—C48—C49 | −179.2 (3) | C41—P2—C35—C40 | −128.7 (4) |
| P3—C47—C52—C51 | −179.3 (3) | C41—C42—C43—C44 | −0.1 (7) |
| P3—C53—C54—C55 | 177.3 (3) | C42—C41—C46—C45 | 0.2 (7) |
| P3—C53—C58—C57 | −175.9 (3) | C42—C43—C44—C45 | −1.1 (8) |
| P3—C59—C60—C61 | 179.5 (4) | C43—C44—C45—C46 | 1.8 (7) |
| P3—C59—C64—C63 | −179.3 (4) | C44—C45—C46—C41 | −1.3 (7) |
| C1—C2—C3—Fe1 | −55.2 (3) | C46—C41—C42—C43 | 0.5 (7) |
| C1—C2—C3—O3 | 134.0 (6) | C47—P3—C53—C54 | 161.5 (3) |
| C1—C2—C4—S1 | −79.3 (5) | C47—P3—C53—C58 | −21.8 (4) |
| C2—C1—C12—S2 | −172.5 (3) | C47—P3—C59—C60 | 111.6 (4) |
| C3—C2—C4—S1 | 111.4 (4) | C47—P3—C59—C64 | −68.5 (4) |
| C4—S1—C5—C6 | 72.5 (4) | C47—C48—C49—C50 | −2.6 (7) |
| C4—S1—C5—C10 | −108.5 (4) | C48—C47—C52—C51 | −2.3 (6) |
| C4—C2—C3—Fe1 | 115.5 (4) | C48—C49—C50—C51 | −0.5 (7) |
| C4—C2—C3—O3 | −55.3 (8) | C49—C50—C51—C52 | 2.1 (7) |
| C5—S1—C4—C2 | −78.1 (4) | C50—C51—C52—C47 | −0.6 (7) |
| C5—C6—C7—C8 | −1.0 (8) | C52—C47—C48—C49 | 3.9 (6) |
| C6—C5—C10—C9 | −0.9 (7) | C53—P3—C47—C48 | 60.8 (4) |
| C6—C7—C8—C9 | −0.2 (8) | C53—P3—C47—C52 | −122.4 (3) |
| C6—C7—C8—C11 | 179.4 (5) | C53—P3—C59—C60 | 0.3 (4) |
| C7—C8—C9—C10 | 0.8 (8) | C53—P3—C59—C64 | −179.9 (4) |
| C8—C9—C10—C5 | −0.3 (8) | C53—C54—C55—C56 | −0.3 (7) |
| C10—C5—C6—C7 | 1.6 (7) | C54—C53—C58—C57 | 0.7 (6) |
| C11—C8—C9—C10 | −178.7 (5) | C54—C55—C56—C57 | −0.9 (7) |
| C12—S2—C13—C14 | −36.3 (5) | C55—C56—C57—C58 | 1.9 (7) |
| C12—S2—C13—C18 | 146.7 (4) | C56—C57—C58—C53 | −1.9 (7) |
| C12—C1—C2—Fe1 | 124.0 (4) | C58—C53—C54—C55 | 0.4 (6) |
| C12—C1—C2—C3 | 173.6 (4) | C59—P3—C47—C48 | −44.9 (4) |
| C12—C1—C2—C4 | 3.3 (7) | C59—P3—C47—C52 | 131.8 (3) |
| C13—S2—C12—C1 | −166.4 (3) | C59—P3—C53—C54 | −93.2 (3) |
| C13—C14—C15—C16 | −1.5 (8) | C59—P3—C53—C58 | 83.5 (4) |
| C14—C13—C18—C17 | −1.1 (7) | C59—C60—C61—C62 | −0.3 (8) |
| C14—C15—C16—C17 | 0.6 (7) | C60—C59—C64—C63 | 0.6 (7) |
| C14—C15—C16—C19 | 179.8 (5) | C60—C61—C62—C63 | 0.8 (8) |
| C15—C16—C17—C18 | −0.1 (7) | C61—C62—C63—C64 | −0.6 (8) |
| C16—C17—C18—C13 | 0.4 (8) | C62—C63—C64—C59 | −0.1 (8) |
| C18—C13—C14—C15 | 1.7 (7) | C64—C59—C60—C61 | −0.4 (7) |
| C19—C16—C17—C18 | −179.3 (5) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C15—H15···O1i | 0.93 | 2.67 | 3.316 (6) | 128 |
| C34—H34A···O3 | 0.97 | 2.62 | 3.271 (5) | 125 |
| C39—H39···O3ii | 0.93 | 2.49 | 3.239 (6) | 138 |
Symmetry codes: (i) x, −y+3/2, z−1/2; (ii) −x+1, −y+1, −z+1.
Funding Statement
This work was funded by Deutsche Forschungsgemeinschaft grant . Verband der Chemischen Industrie grant .
References
- Agilent (2014). CrysAlis PRO. Agilent Technologies, Yarnton, England.
- Akabori, S., Kumagai, T., Shirahige, T., Sato, S., Kawazoe, K., Tamura, C. & Sato, M. (1987). Organometallics, 6, 526–531.
- Akita, M., Sugimoto, S., Terada, M. & Moro-oka, Y. (1993). J. Organomet. Chem. 447, 103–106.
- Aly, S. M., Pam, A., Khatyr, A., Knorr, M., Rousselin, Y., Kubicki, M. M., Bauer, J. O., Strohmann, C. & Harvey, P. D. (2014). J. Inorg. Organomet. Polym. 24, 190–200.
- Amouri, H., Da Silva, C., Malézieux, B., Andrés, R., Vaissermann, J. & Gruselle, M. (2000). Inorg. Chem. 39, 5053–5058. [DOI] [PubMed]
- Bai, S.-Q., Hoi Ka Wong, I., Zhang, N., Lin Ke, K., Lin, M., Young, D. J. & Hor, T. S. A. (2018). Dalton Trans. 47, 16292–16298. [DOI] [PubMed]
- Bennett, S. C., Gelling, A. & Went, M. J. (1992). J. Organomet. Chem. 439, 189–199.
- Boni, A., Funaioli, T., Marchetti, F., Pampaloni, G., Pinzino, C. & Zacchini, S. (2011). J. Organomet. Chem. 696, 3551–3556.
- Bonnot, A., Knorr, M., Strohmann, C., Golz, C., Fortin, D. & Harvey, P. D. (2015). J. Inorg. Organomet. Polym. 25, 480–494.
- Braunstein, P., Knorr, M., DeCian, A. & Fischer, J. (1995). Organometallics, 14, 1302–1309.
- Brieger, L., Jourdain, I., Knorr, M. & Strohmann, C. (2019). Acta Cryst. E75, 1902–1906. [DOI] [PMC free article] [PubMed]
- Casey, C. P., Ha, Y. & Powell, D. R. (1994). J. Am. Chem. Soc. 116, 3424–3428.
- Dennett, J. N. L., Knox, S. A. R., Anderson, K. M., Charmant, J. P. H. & Orpen, A. G. (2005). Dalton Trans. pp. 63–73. [DOI] [PubMed]
- Dickson, R. S., Gatehouse, B. M., Nesbit, M. C. & Pain, G. N. (1981). J. Organomet. Chem. 215, 97–109.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Everhardus, R. H. & Brandsma, L. (1978). Synthesis, pp. 359.
- Fontaine, X. L. R., Jacobsen, G. B., Shaw, B. L. & Thornton-Pett, M. (1988). J. Chem. Soc. Dalton Trans. pp. 741–750.
- Gelling, A., Went, M. J. & Povey, D. C. (1993). J. Organomet. Chem. 455, 203–210.
- Hitchcock, P. B., Madden, T. J. & Nixon, J. F. (1993). J. Organomet. Chem. 463, 155–162.
- Horiuchi, S., Murase, T. & Fujita, M. (2012). Angew. Chem. Int. Ed. 51, 12029–12031. [DOI] [PubMed]
- Johnson, K. A. & Gladfelter, W. L. (1991). J. Am. Chem. Soc. 113, 5097–5099.
- Jourdain, I., Knorr, M., Strohmann, C., Unkelbach, C., Rojo, S., Gómez-Iglesias, P. & Villafañe, F. (2013). Organometallics, 32, 5343–5359.
- Jourdain, I., Vieille-Petit, L., Clément, S., Knorr, M., Villafañe, F. & Strohmann, C. (2006). Inorg. Chem. Commun. 9, 127–131.
- Kiel, G.-Y., Zhang, Z., Takats, J. & Jordan, R. B. (2000). Organometallics, 19, 2766–2776.
- Knorr, M., Guyon, F., Khatyr, A., Däschlein, C., Strohmann, C., Aly, S. M., Abd-El-Aziz, A. S., Fortin, D. & Harvey, P. D. (2009). Dalton Trans. pp. 948–955. [DOI] [PubMed]
- Knorr, M. & Jourdain, I. (2017). Coord. Chem. Rev. 350, 217–247.
- Knox, S. A. R., Lloyd, B. R., Morton, D. A. V., Orpen, A. G., Turner, M. L. & Hogarth, G. (1995). Polyhedron, 14, 2723–2743.
- Levanova, E. P., Vakhrina, V. S., Grabel’nykh, V. A., Rozentsveig, I. B., Russavskaya, N. V., Albanov, A. I., Klyba, L. V. & Korchevin, N. A. (2015). Russ. Chem. Bull. 64, 2083–2089.
- McKee, S. D., Krause, J. A., Lunder, D. M. & Bursten, B. E. (1994). J. Coord. Chem. 32, 249–259.
- Mirza, H. A., Vittal, J. J. & Puddephatt, R. J. (1994). Organometallics, 13, 3063–3067.
- Pourcelot, G. & Cadiot, P. (1966). Bull. Soc. Chim. Fr. 9, 3024–3033.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Wong, A., Pawlick, R. V., Thomas, C. G., Leon, D. R. & Liu, L.-K. (1991). Organometallics, 10, 530–532.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989020007859/pk2630sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989020007859/pk2630Isup3.hkl
CCDC reference: 1996804
Additional supporting information: crystallographic information; 3D view; checkCIF report





