Abstract
Background
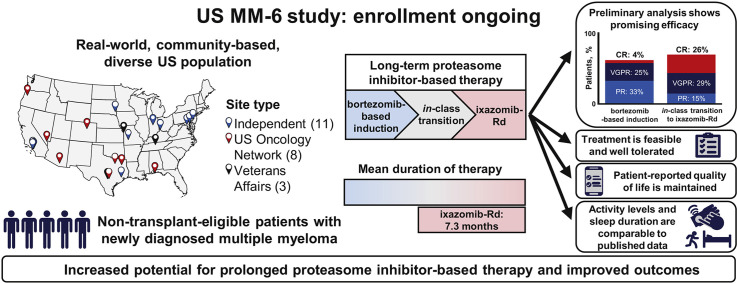
The ongoing US MM-6 study is investigating in-class transition (iCT) from parenteral bortezomib-based induction to all-oral IRd (ixazomib-lenalidomide-dexamethasone) with the aim of increasing proteasome inhibitor (PI)-based treatment adherence and duration while maintaining patients’ health-related quality of life (HRQoL) and improving outcomes.
Patients and Methods
US community sites are enrolling non–transplant-eligible patients with newly diagnosed multiple myeloma (MM) with no evidence of progressive disease after 3 cycles of bortezomib-based therapy to receive IRd (up to 39 cycles or until progression or toxicity). The patients use mobile or wearable digital devices to collect actigraphy (activity and sleep) data and electronically complete HRQoL, treatment satisfaction and medication adherence questionnaires. The primary endpoint is progression-free survival. The key secondary endpoints include response rates and therapy duration.
Results
At the data cutoff, 84 patients had been treated (median age 73 years; 44% aged ≥ 75 years; 49% men; 15% Black or African American; and 10% Hispanic or Latino). Of the 84 patients, 62% were continuing therapy. The mean duration of total PI therapy was 10.1 months and for the IRd regimen was 7.3 months. With an 8-month median follow-up, the 12-month progression-free survival rate was 86% (95% confidence interval, 73%-93%) from both the start of bortezomib-based treatment and the start of IRd. The overall response rate was 62% (complete response, 4%; very good partial response, 25%; partial response, 33%) after bortezomib-based induction and 70% (complete response, 26%; very good partial response, 29%; partial response, 15%) after iCT. The IRd safety profile was consistent with previous clinical trial data, and HRQoL and treatment satisfaction were maintained.
Conclusion
The patients included in the US MM-6 study are representative of the real-world US MM population. The use of iCT might permit prolonged PI-based therapy with promising efficacy, without impacting patients’ HRQoL or treatment satisfaction.
Keywords: Duration of treatment, Medication adherence, Oral therapy, Patient-reported outcomes, Real-world community
Graphical abstract
Micro-Abstract
Although long-term proteasome inhibitor therapy improves outcomes in multiple myeloma, many patients cannot tolerate long-term treatment or might require or prefer to continue treatment outside the hospital or clinic. The US MM-6 study is evaluating the in-class transition from parenteral bortezomib- to oral ixazomib-based therapy in routine clinical practice. Preliminary results indicate feasibility, prolonged therapy duration, promising efficacy, and treatment adherence and satisfaction.
Introduction
Proteasome inhibitors (PIs) have been the cornerstone of treatment for transplant-eligible and -ineligible patients with newly diagnosed multiple myeloma (NDMM) and for those with previously treated disease.1 For transplant-ineligible patients, compared with non–PI-based therapy, PI-based therapy has improved both progression-free survival (PFS) and overall survival (OS) in global phase III randomized controlled trials.2, 3, 4 Additionally, data have shown that longer, continuous PI-based therapy results in prolonged PFS and OS compared with shorter, fixed-duration therapy.5 , 6 However, the real-world outcomes have often not matched those obtained in clinical studies,7, 8, 9, 10 and the median treatment duration for PI-based therapy has often been shorter in routine clinical practice than in clinical trials.7 , 9 , 11
This disparity may be due to various factors, including older patient age, a high comorbidity burden, socioeconomic status, ethnicity/racial differences, poor treatment adherence, burden of repeated intravenous or subcutaneous administration (which can negatively impact patients’ health-related quality of life [HRQoL]12), cost considerations, and toxicity (eg, peripheral neuropathy [PN] with bortezomib, which can worsen with prolonged exposure, potentially leading to treatment discontinuation2 , 13).7 Additionally, many reasons can make it difficult for patients to travel to or have access to infusion centers to receive treatment at a clinic (eg, environmental conditions, travel restrictions, social/family situations). Also, some patients might prefer to continue treatment outside of a hospital or clinic setting.
Patients have often been excluded from clinical trials because of many of the factors listed in the previous paragraph, raising concerns that real-world patients have been underrepresented in clinical trials.7 Analyses have shown that up to 40% of real-world patients with NDMM would be ineligible for participation in clinical trials.8 , 14 , 15 Given that extended treatment with PI-based therapy has resulted in improved outcomes in clinical trials,5 we believed it would be valuable to conduct a study to assess the potential benefit of continuous, long-term PI-based treatment in a US community setting. A novel approach to facilitate long-term PI-based treatment in routine clinical practice would be to use an in-class transition (iCT) from a parenteral (bortezomib) to an oral (ixazomib) PI. Parental bortezomib-based therapy is given as induction during early treatment cycles, when more frequent clinic visits might be required for close disease monitoring, followed by subsequent iCT to all-oral ixazomib-based therapy in later cycles to improve convenience during the longer term, outpatient-based management. The iCT is made possible by the availability of ixazomib, an oral, once-weekly, boron-based, reversible PI,16 which has been approved for use in combination with Rd (lenalidomide, dexamethasone) for treatment of patients with MM who have received ≥ 1 prior therapy.17 The feasibility of long-term ixazomib-Rd (IRd) treatment for patients with relapsed/refractory MM has been demonstrated by both clinical trial and real-world data. The real-world findings18, 19, 20, 21, 22, 23, 24 have suggested that the effectiveness of extended treatment with IRd appears similar to the efficacy reported in clinical trials25 and that the combination is well tolerated, with low rates of grade 3/4 PN.19 , 20 , 25
The US MM-6 study (an effectiveness and safety study of ixazomib in combination with lenalidomide and dexamethasone in participants with multiple myeloma previously receiving a bortezomib-based induction regimen; ClinicalTrials.gov identifier, NCT03173092) is investigating the novel iCT approach with the aim of increasing PI-based treatment duration and adherence, maintaining HRQoL, and improving outcomes for patients with NDMM, with a study design that allows centers to follow their standard-of-care procedures for first-line bortezomib-based induction therapy. This US-based study will provide valuable interventional, prospective, real-world data for this iCT approach and is using a novel data collection method to evaluate electronic patient-reported outcomes (ePROs) in an entirely community-based setting, including patients from racial/ethnic minorities. In the present report, we have addressed the feasibility of performing such a trial exclusively in the US community setting and reported the preliminary data from the first 84 patients enrolled and treated in the ongoing US MM-6 study.
Patients and Methods
Study Design and Patients
US MM-6 is a community-based, real-world, open-label, single-arm phase IV study. Eligible patients are adults with NDMM (using the International Myeloma Working Group criteria26) who do not meet the transplant-eligibility criteria and those for whom transplantation would be delayed for ≥ 24 months. Also, the patients must be receiving first-line bortezomib-based induction (in accordance with the regimens listed in US National Comprehensive Cancer Network guidelines) and have no evidence of progressive disease (PD) after 3 treatment cycles. Patients are enrolled within 14 days of completing the third bortezomib-based induction cycle. At that time, they must have an Eastern Cooperative Oncology Group and/or other performance status of 0 to 2 and no grade ≥ 2 PN or grade 1 PN with pain to be eligible. Stem cell harvest and mobilization are allowed, if clinically indicated. The eligibility criteria are summarized in Supplemental Appendix 1 (available in the online version). At the time of this datacut patients are being enrolled at 22 US community sites (including 3 Veterans Affairs sites). The patients receive all-oral IRd in 28-day cycles (ixazomib 4 mg on days 1, 8, and 15; lenalidomide 25 mg on days 1-21; and dexamethasone 40 mg [20 mg for patients aged > 75 years] on days 1, 8, 15, and 22) for up to 39 cycles or until PD or toxicity. The patients must continue receiving ixazomib to remain in the study. After an end-of-treatment assessment (within 30 days of the last ixazomib dose), the patients enter a follow-up period for evaluation of PFS and OS until PD or death, loss to follow-up, or study termination by the sponsor. The protocol changes affecting study conduct are summarized in Supplemental Appendix 1 (available in the online version).
US MM-6 is being conducted in accordance with the International Council on Harmonisation Good Clinical Practice guidelines, the ethical principles that have their origins in the Declaration of Helsinki, and applicable regulatory requirements. The local or central institutional review boards for each study center approved the present study. All the patients gave written informed consent.
Endpoints and Assessments
The primary endpoint is 2-year PFS (defined as the time from the first administration of IRd to the first documentation of PD or death from any cause). Key secondary endpoints are the response rates, duration of response, duration of therapy (IRd and ixazomib), and relative dose intensity for each study drug (the endpoint definitions are provided in Supplemental Appendix 1; available in the online version). Additional secondary endpoints include safety and PFS for patients carrying del(17), t(4;14), or t(14;16). Additional secondary and exploratory endpoints capture patients’ experiences in the real-world community setting. Wearable digital and mobile devices to collect actigraphy (activity and sleep) data and ePROs to assess HRQoL, treatment satisfaction, and medication adherence are being used.
Response and disease progression are being evaluated by investigators according to the modified International Myeloma Working Group response criteria27 and per the regular clinical practice of the treating physician. The safety of IRd is being assessed via adverse event (AE) monitoring from the first dose through 30 days after the last dose of the study drug regimen (IRd). Toxicity is being evaluated using the Medical Dictionary for Regulatory Activities (version 22.0) preferred terms and graded according to the National Cancer Institute Common Terminology Criteria for AEs (version 4.03). Medication adherence, HRQoL, and treatment satisfaction are being assessed via ePRO instruments, which patients complete every cycle during IRd treatment and at the end-of-treatment assessment visit using mobile devices. Starting with cycle 2, ePROs are manually launched by site staff at approximately on day 1 of each IRd cycle; once launched, patients have a 7-day window for ePRO completion. Thus, the first ePRO assessment (ePRO baseline) occurs at the end of cycle 1. The instruments to complete are the following: daily and monthly medication adherence questionnaires; the European Organization for Research and Treatment of Cancer (EORTC) core QoL questionnaire (QLQ-C30; version 3; Global Health Status/QoL scale [items 29 and 30]); the EORTC QoL questionnaire-MM module (QLQ-MY20; PN [item 43]); and the Treatment Satisfaction Questionnaire for Medication (TSQM-9; subscale scores “effectiveness,” “convenience,” and “global satisfaction”; the scoring details are provided in Supplemental Appendix 1; available in the online version). Patients self-report their monthly medication adherence using a choice of provided categories (excellent, very good, good, fair, or poor) to answer the question “Thinking about the past 4 weeks, please rate your ability to take your oral cancer medication as prescribed.” Patients wear digital devices (Garmin Vivofit 3 activity tracker) to capture the actigraphy data (activity [steps, distance] and sleep time [hours] daily) during IRd treatment.
Statistical Analysis
The planned enrollment is ∼160 patients. The sample size was determined, assuming a 10% loss-to-follow-up rate, to provide 90% power at an α of 0.05 to demonstrate trial success with a 2-year PFS rate of > 57%, based on an assumption of a 2-year PFS rate of 62% for the present study and an estimated 2-year PFS rate of 50% derived from historical controls.2 , 28 , 29 The treatment phase is expected to last for 78 months, with a 42-month enrollment period and 36-month IRd treatment period from the date of last patient enrolled. The safety population comprises all patients who receive ≥ 1 dose of the study drug regimen (IRd). All enrolled patients are included in the intent-to-treat population. Further details regarding the specific data analyses are provided in Supplemental Appendix 1 (available in the online version). Data analysis was performed by the sponsor, contract research organization, steering committee, and authors. All the authors had access to the primary clinical trial data.
Results
Patients and Treatment
As of November 18, 2019, 84 patients had been enrolled and received ≥ 1 dose of the study drug regimen (IRd). Of these 84 patients, 44% were aged ≥ 75 years, 15% were Black or African American, 10% were Hispanic or Latino, 29% had a creatinine clearance < 60 mL/min, and 99% had any concurrent medical condition (Table 1 ). The most common induction regimen at iCT to IRd was VRD (bortezomib, lenalidomide, dexamethasone; 85%); 13% of patients were receiving VCD (bortezomib, cyclophosphamide, dexamethasone) and 2% were receiving other bortezomib-containing regimens (VD [bortezomib, dexamethasone] or VR [bortezomib, lenalidomide]; Table 1). Bortezomib was administered once and twice weekly for 86% (68 of 79) and 14% (11 of 79) of patients, respectively. The most common dose of bortezomib received was 1.3 mg/m2 (82%; 67 of 82 patients).
Table 1.
Baseline Demographics, Disease Characteristics, and Previous Induction Regimensa
| Characteristic | Patients (n = 84) |
|---|---|
| Age, y | |
| Median | 73 |
| Range | 49-90 |
| Age group, n (%) | |
| ≥65 y | 67 (80) |
| ≥75 y | 37 (44) |
| Male gender, n (%) | 41 (49) |
| Race, n (%) | |
| White | 61 (73) |
| Black/African American | 13 (15) |
| Asian | 2 (2) |
| >1 Race selected | 1 (1) |
| Missing | 7 (8) |
| Ethnicity, n (%) | |
| Hispanic/Latino | 8 (10) |
| Not Hispanic/Latino | 72 (86) |
| Not reported/unknown | 4 (5) |
| ISS stage at initial diagnosis, n (%) | |
| I | 22 (26) |
| II | 25 (30) |
| III | 29 (35) |
| Unknown | 8 (10) |
| Type of myeloma at initial diagnosis, n (%) | |
| Heavy chain | |
| IgG | 50 (60) |
| IgA | 16 (19) |
| IgD | 1 (1) |
| IgM | 1 (1) |
| Multiple | 7 (8) |
| Missing | 9 (11) |
| Light chain | |
| Kappa | 51 (61) |
| Lambda | 27 (32) |
| Multiple | 5 (6) |
| Missing | 1 (1) |
| Lytic bone disease, n (%) | 35 (42) |
| Extramedullary disease, n (%) | 6 (7) |
| Creatinine clearance,b mL/min | |
| Median | 69 |
| Range | 12-226 |
| Calculated creatinine clearance, n (%) | |
| <30 mL/min | 4 (5) |
| 30 to < 60 mL/min | 20 (24) |
| 60 to < 90 mL/min | 35 (42) |
| ≥90 mL/min | 17 (20) |
| Missing | 8 (10) |
| Concurrent medical conditions,c n (%) | 83 (99) |
| Hypertension | 48 (57) |
| Anemia | 37 (44) |
| Fatigue | 36 (43) |
| Renal/urinary disordersd | 34 (40) |
| Cardiac disordersd | 25 (30) |
| Insomnia | 25 (30) |
| Gastroesophageal reflux disease | 24 (29) |
| Constipation | 24 (29) |
| Back pain | 19 (23) |
| Nausea | 17 (20) |
| Peripheral edema | 17 (20) |
| Anxiety | 16 (19) |
| Hyperlipidemia | 15 (18) |
| Arthralgia | 14 (17) |
| Hypercholesterolemia | 13 (15) |
| Peripheral neuropathy | 11 (13) |
| Diabetes mellituse | 11 (13) |
| Induction regimen at the time of iCT to IRd, n (%) | |
| VRD | 71 (85) |
| VCD | 11 (13) |
| Other (VD; VR) | 2 (2) |
Abbreviations: iCT = in-class transition; IRd = ixazomib, lenalidomide, dexamethasone; ISS = International Staging System; VCD = bortezomib, cyclophosphamide, dexamethasone; VD = bortezomib, dexamethasone; VR = bortezomib, lenalidomide; VRD = bortezomib, lenalidomide, dexamethasone.
At enrollment (or initial diagnosis for ISS stage and type of myeloma).
Patient number totaled 76.
Occurring in ≥ 15% of patients and specific other comorbidities of clinical importance.
System organ class (other concurrent medical conditions listed by preferred term).
Included preferred terms of diabetes mellitus (n = 4) and type 2 diabetes mellitus (n = 7).
The mean duration of PI therapy, from the start of bortezomib-based induction, was 10.1 months (Table 2 ). The mean duration of IRd and ixazomib was 7.3 months and 7.0 months, respectively. Patients have received up to 23.0 months of treatment with IRd at data cutoff. At the data cutoff for the 84 patients, 52 (62%) were continuing therapy and 32 (38%) had discontinued treatment. Treatment was discontinued because of PD (n = 5), AEs (n = 4), and other reasons (n = 23). The most common other reason for discontinuation was patient and/or physician decision or withdrawal of consent (n = 15; other reasons included sufficient response, n = 3; hospitalization, n = 2; and inadequate response, death, and no reason, n = 1 for each).
Table 2.
Treatment Exposurea
| Variable | Patients (n = 84) |
|---|---|
| PI therapy duration, including bortezomib-based induction,b mo | |
| Mean ± SD | 10.1 ± 5.6 |
| Medianc | 8.8 |
| Range | 2.6-26.4 |
| IRd therapy duration,d mo | |
| Mean ± SD | 7.3 ± 5.7 |
| Medianc | 6.1 |
| Range | 0.03-23.0 |
| Ixazomib therapy duration,e mo | |
| Mean ± SD | 7.0 ± 5.8 |
| Medianc | 5.1 |
| Range | 0.03-22.8 |
| Lenalidomide therapy duration,e mo | |
| Mean ± SD | 7.3 ± 5.7 |
| Medianc | 6.0 |
| Range | 0.03-23.0 |
| Relative dose intensity,f % (mean ± SD) | |
| Ixazomib | 77 ± 31 |
| Lenalidomide | 80 ± 89 |
| Dexamethasone | 85 ± 56 |
| Patients with no. of IRd treatment cycles, n (%) | |
| ≥3 | 67 (80) |
| ≥6 | 46 (55) |
| ≥12 | 19 (23) |
| ≥18 | 9 (11) |
| ≥24 | 1 (1) |
Abbreviations: IRd = ixazomib, lenalidomide, dexamethasone; PI = proteasome inhibitor; SD = standard deviation.
Patients had to continue receiving ixazomib to remain in the study; a dose interruption of ixazomib lasting > 3 weeks or an interruption at the principal investigator’s discretion was considered discontinuation from ixazomib treatment and the patient was discontinued from the study, although they could be followed for progression-free and overall survival. In addition, at the discretion of the treating physician, patients could discontinue treatment from lenalidomide and/or dexamethasone but continue ixazomib treatment and remain in the study.
Time from the date of first administration of the bortezomib-based induction regimen to the date of the last administration of ixazomib, lenalidomide, or dexamethasone.
Simple median (ie, not determined using Kaplan-Meier method).
Time from the date of the first administration of IRd to the date of the last administration of ixazomib, lenalidomide, or dexamethasone.
Time from the date of the first administration of ixazomib, lenalidomide to the date of the last administration of ixazomib, lenalidomide.
Relative dose intensity for each study drug was defined as 100 × (total amount of dose taken)/(total prescribed dose of treated cycles), where the total prescribed dose equaled the (dose prescribed at enrollment × the number of prescribed doses per cycle × the number of treated cycles).
Efficacy
After a median follow-up of 8 months, and with enrollment ongoing, 6 patients had experienced progression and 2 patients had died. The preliminary 12-month PFS rate was 86% (95% confidence interval, 73%-93%) both from the start of bortezomib-based induction and from the start of IRd treatment.
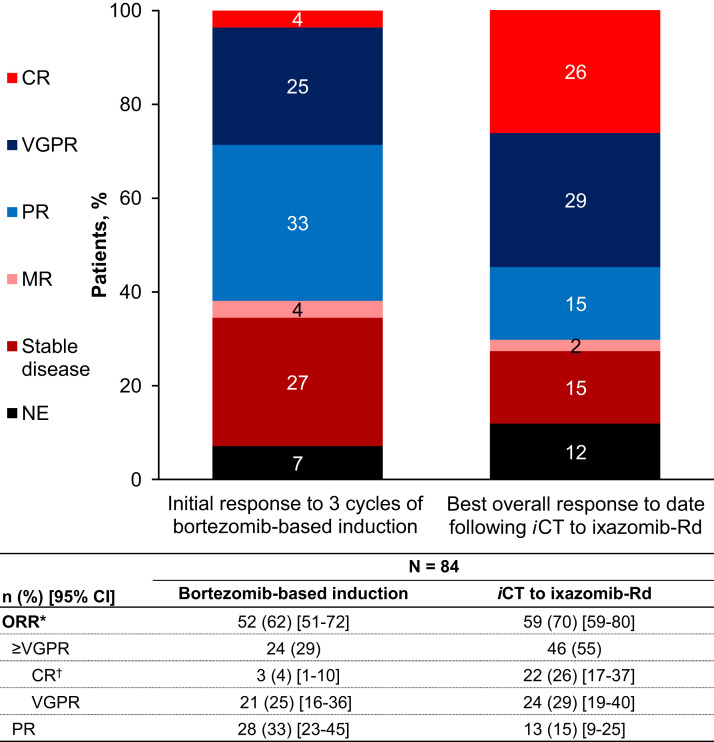
The overall response rate (ORR) after bortezomib-based induction (before study start) was 62%, with 4%, 25%, and 33% of patients achieving a complete response (CR), very good partial response (VGPR), and partial response (PR), respectively, as their best response. After the iCT to IRd, the ORR increased to 70%, with the CR and VGPR rates increasing to 26% and 29%, respectively (Figure 1 ).
Figure 1.
Investigator-assessed Best Overall Responses After Bortezomib-based Induction and After an in-Class Transition (iCT) to IRd (Ixazomib, Lenalidomide, Dexamethasone). ∗Overall Response Rate (ORR) Included Complete Response (CR), Very Good Partial Response (VGPR), and Partial Resonse (PR). †CR Category Included Patients With a CR, Stringent CR, Immunophenotypic CR, or Molecular CR
Abbreviations: CI = confidence interval; MR = minimal response; NE = not evaluable.
Safety
The safety profile of IRd is summarized in Table 3 . During the treatment to date, any grade treatment-emergent AEs (TEAEs) were reported for 92% of patients. The most common (> 20%) any grade TEAEs were diarrhea, PN not elsewhere classified (high-level term), fatigue, and nausea. The most common (≥ 5%) grade 3 TEAEs were diarrhea, pneumonia, syncope, and anemia (Table 4 ). Grade 4 TEAEs occurred in 6 patients; by preferred term, these were cardiac failure, atrial fibrillation, hypercalcemia, metabolic acidosis, septic shock, decreased neutrophil count, decreased white blood cell count, metabolic encephalopathy, and pulmonary embolism (n = 1 for each). AEs led to dose adjustments of ixazomib, lenalidomide, and dexamethasone in 39%, 39%, and 29% of patients, respectively. Specifically, the TEAEs that led to dose modifications for any of the 3 study drugs included diarrhea (8% of patients), PN (8%), nausea (5%), cellulitis (4%), decreased neutrophil count (4%), fatigue (4%), and pneumonia (4%). The only serious TEAE occurring in > 2 patients was pneumonia (5%). Of the 84 patients, 2 have died during study participation; 1 died of end-stage renal disease that was not treatment-related and 1 of pneumonia, which was treatment-related.
Table 3.
Overview of Safety Profile of IRd (n = 84)
| TEAEa | Patients, n (%) |
|---|---|
| Any grade TEAE | 77 (92) |
| Any grade treatment-related TEAE | 59 (70) |
| Grade ≥ 3 TEAE | 40 (48) |
| Grade ≥ 3 treatment-related TEAE | 21 (25) |
| TEAE leading to drug modificationb | 42 (50) |
| TEAE leading to drug discontinuationb | 6 (7) |
| Serious TEAE | 30 (36) |
| Treatment-related serious TEAE | 9 (11) |
| Deaths during study | 2 (2) |
Abbreviations: IRd = ixazomib, lenalidomide, dexamethasone; TEAE = treatment-emergent adverse event.
TEAEs were defined as adverse events that occurred after administration of the first dose through 30 days after the last dose of the study drug regimen (IRd). TEAEs were considered serious if they resulted in death, were life-threatening, had required inpatient hospitalization or prolongation of an existing hospitalization (excluding planned hospital admissions and surgical procedures for a preexisting condition unless it had deteriorated unexpectedly during the study period), had resulted in persistent or significant disability or incapacity, or were a congenital anomaly/birth defect or a “medically important event”.
Modification or discontinuation of any of the 3 study drugs (ixazomib, lenalidomide, dexamethasone).
Table 4.
Frequently Occurring Treatment-emergent Adverse Events During IRd Treatmenta (n = 84)
| TEAE | Grade, n (%) |
|
|---|---|---|
| Any Grade | Grade 3 | |
| Diarrhea | 34 (40) | 6 (7) |
| PN NECb | 25 (30) | 2 (2) |
| Fatigue | 20 (24) | 2 (2) |
| Nausea | 20 (24) | 2 (2) |
| Back pain | 16 (19) | 1 (1) |
| Constipation | 14 (17) | 0 (0) |
| Hypokalemia | 13 (15) | 2 (2) |
| Cough | 13 (15) | 0 (0) |
| Dizziness | 12 (14) | 1 (1) |
| Peripheral edema | 12 (14) | 0 (0) |
| Anemia | 11 (13) | 4 (5) |
| Arthralgia | 11 (13) | 0 (0) |
| Hypotension | 10 (12) | 2 (2) |
| Rash | 10 (12) | 2 (2) |
| Pneumonia | 9 (11) | 5 (6) |
| Vomiting | 9 (11) | 2 (2) |
| Decreased appetite | 8 (10) | 1 (1) |
| Decreased platelet count | 8 (10) | 1 (1) |
| Pyrexia | 8 (10) | 0 (0) |
| Syncope | 5 (6) | 5 (6) |
Abbreviations: IRd = ixazomib, lenalidomide, dexamethasone; PN NEC = peripheral neuropathy, not elsewhere classified.
Cutoffs for inclusion were ≥ 10% of patients with any grade or ≥ 5% of patients with grade 3 in the intent-to-treat population.
High-level term.
Electronic PROs
At the data cutoff, the EORTC QLQ-C30, EORTC QLQ-MY20, TSQM-9, and monthly medication adherence questionnaires had been completed by 97.3%, 97.6%, 98.7%, and 97.4% of the patients who had received these instruments, respectively (Supplemental Appendix 1; available in the online version).
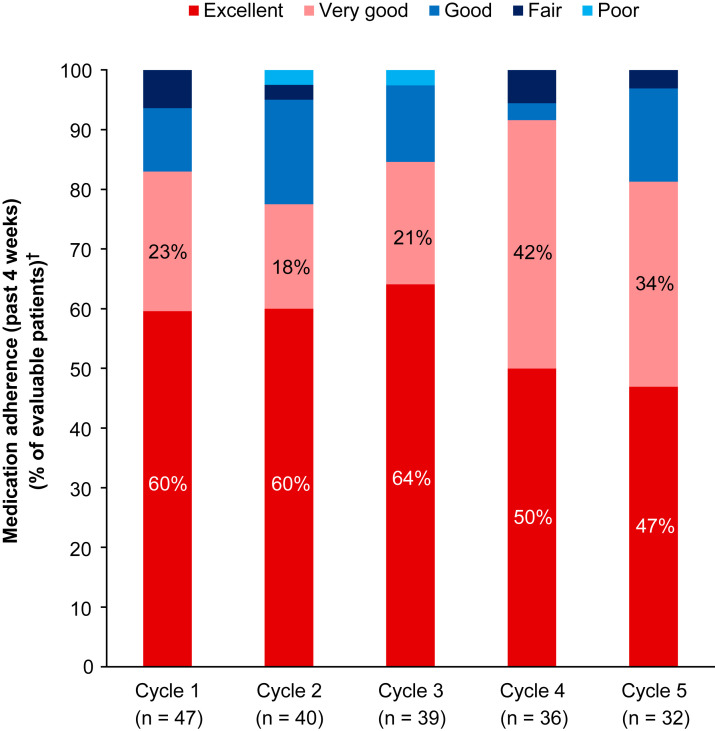
The patient-reported monthly medication adherence for the previous 4 weeks was reported as excellent or very good by 78% to 92% of patients who had reported medication adherence in cycles 1 to 5 (Figure 2 ). The most common patient-reported reason for not taking the medication was the development of side effects (range, 6%-19%). Monthly ePRO medication adherence data have been recorded for a maximum of 24 cycles at the data cutoff date. After cycle 5, the number of evaluable patients was < 30% (data available for 1-22 patients for cycles 6-24; n = 1 in cycle 24).
Figure 2.
Patient-Reported Medication Adherence (Ixazomib, Lenalidomide, Dexamethasone) Over Time (Cycles 1-5). Patients Had Self-Reported Their Monthly Medication Adherence Using a Choice of Provided Categories (Excellent, Very Good, Good, Fair, or Poor) to Answer the Question “Thinking About the Past 4 Weeks, Please Rate Your Ability to Take Your Oral Cancer Medication as Prescribed?” After Cycle 5, the Number of Evaluable Patients Was < 30%. The Maximum Number of Cycles Received at the Data Cutoff Was 25 (n = 1). Patient-Reported Monthly Medication Adherence Data for the Previous 4 Weeks Were Available Through to Cycle 24 (n = 1). †Percentages Were Calculated According to the Number of Patients Who Had Reported Medication Adherence in Each Cycle
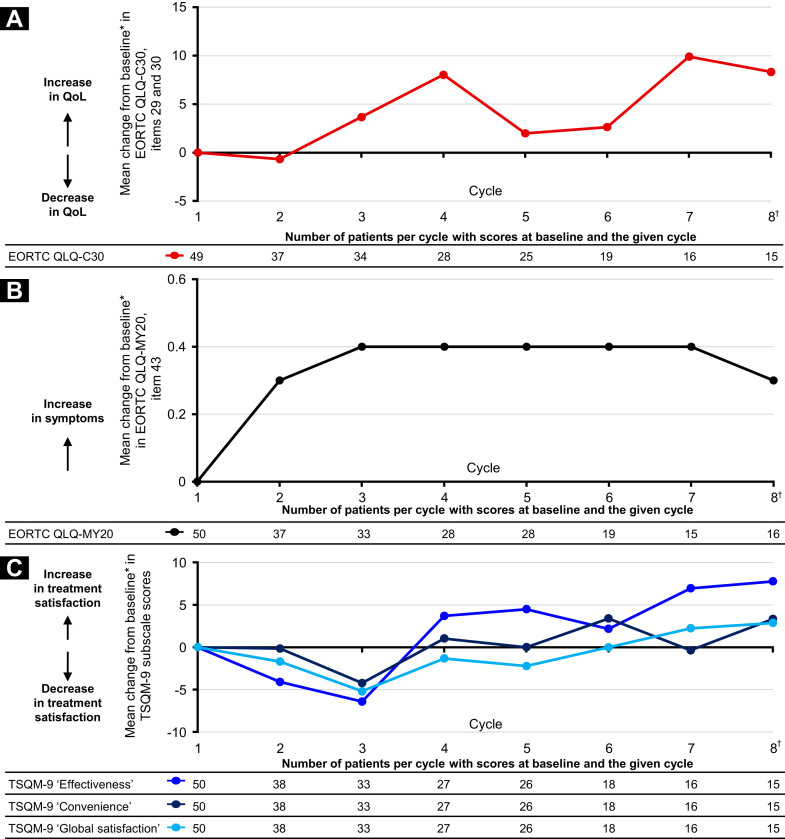
At data cutoff, the maximum number of patients for whom EORTC QLQ-C30, EORTC QLQ-MY20, and TSQM-9 scores were available at ePRO baseline and in any given later cycle was 49, 50, and 50, respectively. During IRd treatment (through cycle 8, beyond which the numbers of patients with ePRO data were ≤ 10), the changes from ePRO baseline in global health status/QoL and treatment satisfaction were relatively small, indicating that both were maintained for those patients for whom data were available (Figure 3 ). The mean change from ePRO baseline on the EORTC QLQ-MY20 PN item was ≤ 0.4 through cycle 8.
Figure 3.
Electronic Patient-Reported Outcomes (ePROs) During Treatment With IRd (Ixazomib, Lenalidomide, Dexamethasone). (A) Mean Change From ePRO Baseline in European Organization for the Research and Treatment of Cancer (EORTC) Core Quality of Life (QoL) Questionnaire (QLQ-C30) Global Health Status/QoL Subscale Score (Items 29 and 30). (B) Mean Change From ePRO Baseline in EORTC QoL Questionnaire–Multiple Myeloma Module (QLQ-MY20) Item 43 (Peripheral Neuropathy). (C) Mean Change From ePRO Baseline in Treatment Satisfaction Questionnaire for Medication (TSQM)-9 Subscale Scores. ∗ePRO Baseline Was Defined as Reported Measurement at End of Cycle 1 of IRd; Change From ePRO Baseline Was Only Calculated at Post-ePRO Baseline IRd Cycles for Which a Value Was Present and for Patients With an ePRO Baseline Value. †Data Were Available for up to 24 Cycles for Each Measure. Data Not Shown After Cycle 8 Because of Small Patient Numbers (n ≤ 10). Global Health Status/QoL Subscale Was Derived From Items 29 and 30 of the EORTC QLQ-C30 (Version 3). This Scale Has a Range of 0 to 100. Positive and Negative Changes Indicate an Improvement and Deterioration, Respectively, in QoL. EORTC QLQ-MY20 Item 43 (“Did You Have Tingling Hands or Feet?”) Measured the Burden of Peripheral Neuropathy Symptoms Using a Score Range of 1 to 4 (1, Not at All; 2, a Little; 3, Quite a Bit; and 4, Very Much). A Higher Score Indicates an Increase in Symptoms. The TSQM-9 Subscales (Effectiveness, Convenience, and Global Satisfaction) Have a Range of 0 to 100. Positive and Negative Changes Indicate an Increase and Decrease, Respectively, in Treatment Satisfaction
Actigraphy
At the data cutoff for actigraphy analysis (November 15, 2019), data were available for 71 patients; 9160 compliant days of actigraphy data had been recorded out of a total of 10064 days during which actigraphy data were available. The mean ± standard deviation (SD) number of steps daily was 2931 ± 2234, the mean ± SD number of meters daily was 2062 ± 1595, and the mean ± SD sleep time was 7.90 ± 2.60 hours daily.
Subgroup Analyses by Age
The analysis results by age (< 75 years [n = 47] vs. ≥ 75 years [n = 37]) are presented in Supplemental Appendix 1 (available in the online version). The baseline demographics and disease characteristics are presented in Supplemental Table 1 (available in the online version). The mean duration of total PI therapy (from the start of bortezomib-based induction) was 10.8 and 9.3 months, and the mean duration of IRd was 8.0 and 6.4 months, in the younger and older subgroups, respectively (Supplemental Table 2; available in the online version). The ORR after bortezomib-based induction was 57% (including 2% with a CR) for patients aged < 75 years and 68% (5% with a CR) in those aged ≥ 75 years. After the iCT to IRd, the ORR was 68% (30% with a CR) for patients aged < 75 years and 73% (22% with a CR) for those aged ≥ 75 years (Supplemental Table 3; available in the online version). Grade ≥ 3 TEAEs were reported in 45% and 51% of the younger and older patients, respectively (Supplemental Table 4; available in the online version). The most common (≥ 25% in either subgroup) any-grade TEAEs were diarrhea, PN not elsewhere classified, fatigue, nausea, and back pain (Supplemental Table 5; available in the online version). ePRO and actigraphy data were not analyzed by age because of insufficient patient numbers.
Discussion
This early snapshot of the prospective, interventional US MM-6 study investigated the feasibility of a novel iCT strategy developed to allow real-world, non-transplant-eligible patients with MM from US community centers to benefit from long-term PI-based therapy. The population for the ongoing US MM-6 study includes patients from the community who might not be eligible for clinical trials because of factors that could impact their ability to respond to and/or tolerate treatment, such as older age, poor performance status, advanced disease stage, prevalent comorbidities, and laboratory abnormalities (which could indicate neutropenia, thrombocytopenia, anemia, hypercalcemia, or poor renal or hepatic function).8 , 14 , 15 , 30 Renal impairment is a common presenting feature in patients with NDMM, and patients with renal impairment have been underrepresented in randomized clinical trials owing to exclusion criteria that include the creatinine clearance level.15 Several of the enrollment criteria used in US MM-6 allow for inclusion of a broader patient population compared with the criteria used in standard clinical trials (eg, all creatinine clearance levels were permitted, a history of previous malignancies > 2 years previously was permitted, and iCT was permitted for patients achieving only stable disease after bortezomib-based induction therapy). Furthermore, the study was designed to enroll patients solely from community centers and to allow the centers to follow their own usual standard-of-care procedures for the selection of first-line bortezomib-based induction therapy and patient evaluation and management. The trial was designed with the administration of 3 cycles of bortezomib-based induction therapy to maximize the potential for effective disease control early in treatment, when all patients are closely monitored, and to reduce the potential for progressive side effects (eg, PN) and the treatment burden of long-term parenteral administration. All patients were scheduled for an iCT to IRd if they showed no progression during the initial bortezomib-based therapy. The subsequent iCT to IRd allows for continued PI therapy with an all-oral triplet approach, conducive to outpatient management, with the aims of sustaining and improving efficacy and maintaining a manageable long-term safety profile.
Evaluation of patient-level data from the US-based Connect-MM registry and other analyses of real-world MM populations have shown that 22% to 73% of patients with MM would be ineligible to participate in randomized controlled trials.8 , 14 , 15 , 30 Consequently, efforts are ongoing to broaden the clinical trial inclusion criteria, where possible,7 , 31 with studies such as US MM-6 designed to ensure generalizability of the results to patients who will be receiving anti-MM treatments in everyday practice. To date, in the US MM-6 study, the median age is 73 years, with nearly one half of the patients aged ≥ 75 years and just more than one third with International Staging System stage III disease. Of the 84 patients, 15% were Black or African American and 10% were of Hispanic or Latino ethnicity. The patients also had multiple comorbidities. The baseline characteristics of the US MM-6 patients (including age, comorbidities, and ethnicity) are similar to those reported for transplant-ineligible patients with NDMM in the US community-based phase IIIB UPFRONT trial (a comparison of three frontline bortezomib-based regimens),29 with both studies contributing to the increasing body of evidence obtained for PI-based therapies in more representative real-world populations.
With a median follow-up of 8 months and 62% of treated patients still receiving therapy at the data cutoff, the median duration of total PI therapy (from the start of bortezomib-based induction) was 8.8 months, and the median duration of IRd was 6.1 months. Some of the patients have received up to 25 cycles of treatment. Although the discontinuation rate appears high, given that the US MM-6 data are not yet mature, these findings are promising. A full understanding of the discontinuation rate will require full enrollment and data maturity.
With the study ongoing, the responses have continued to evolve, with ORRs and CR rates increasing after iCT to all-oral IRd. The ORR of 70% after iCT is comparable to the 74% (using the International Uniform Response Criteria) reported for bortezomib plus melphalan and prednisone in the VISTA (Velcade/melphalan/prednisone versus melphalan/prednisone in patients with previously untreated multiple myeloma) study3 (an evaluation of bortezomib plus melphalan and prednisone in patients with previously untreated MM ineligible for high-dose therapy) and the 70% to 80% with bortezomib-based regimens in the UPFRONT study29 for patients with NDMM. PFS data from US MM-6, including in patients with high-risk cytogenetics, were immature at the time of the present analysis.
The IRd safety profile reported at data cutoff for the US MM-6 study is consistent with previous clinical trial data.25 The most commonly occurring TEAEs after iCT included gastrointestinal events and PN. Although patients with grade 1 PN at the time of iCT could be included, the incidence of PN at the data cutoff was in line with that from other studies of ixazomib.25 , 28 , 32, 33, 34 Furthermore, the patients’ mean EORTC QLQ-MY20 score (PN item) was generally stable during treatment, consistent with only a limited increase in PN symptoms during the treatment course with IRd.
The initial analysis of the present data by age indicated the potential for the long-term tolerability of IRd in patients aged < 75 and ≥ 75 years. Thus, it appears that, using an iCT approach, long-term PI-based treatment with IRd is feasible for older patients and could offer patients in real-world settings the same improved efficacy obtained with long-term PI inhibition in clinical studies.
With prolonged outpatient-based treatment, concerns always exist regarding medication adherence and whether patients are maintaining an adequate HRQoL. To monitor these factors, the US MM-6 study is using modern digital solutions to collect ePRO data, including mobile devices to collect data on medication adherence, HRQoL, and treatment satisfaction, and a wearable digital device to record actigraphy data. In the present analysis, the reported ePRO completion proportions were high, indicating that real-world studies using electronic data collection devices are feasible for this mostly elderly, comorbid population. Although at the present analysis, few patients have received later cycles of IRd, for the earlier treatment cycles with higher numbers of evaluable patients, the patient-reported medication adherence is high (ie, excellent or very good for ≥ 78% of patients), indicating the feasibility of patients continuing the all-oral PI-based triplet regimen. The preliminary ePRO data gathered demonstrated no adverse impact of longer term IRd treatment on patients’ HRQoL and treatment satisfaction.
The actigraphy data include activity measured in number of steps daily and sleep duration measured in number of hours daily. The mean number of steps daily reported in US MM-6 is comparable to the reported data for healthy adults aged > 65 years and for adults living with disability and/or chronic illness that might limit mobility and/or physical endurance.35 The mean sleep duration is also comparable to that determined previously in healthy adults (with no major medical disorders) aged ≥ 60 years36 and in patients with MM.37 With longer follow-up, more mature data will be available for analysis in the US MM-6 study. In future MM studies, the potential exists for specific algorithms to be applied to actigraphy data in real time to generate health alerts for particular follow-up by the treating physician.
Conclusion
The US MM-6 study is using a novel iCT approach to facilitate long-term, PI-based treatment for real-world, non-transplant-eligible patients with NDMM. The study has been successfully enrolling a patient population representative of real-world patients. Our findings to date represent a unique, interventional, prospective dataset obtained in the US community setting from mostly elderly patients with comorbidities and including minority racial and ethnic subgroups. In this context, and with enrollment and follow-up ongoing, we have demonstrated that iCT from parenteral bortezomib-based induction to all oral IRd is feasible, allowing for long-term PI-based treatment that is well tolerated with promising efficacy, good medication adherence, and no adverse impact on patients’ HRQoL or treatment satisfaction.
In addition to offering a viable treatment option for underrepresented patient populations, the ability to transition from a parenteral to an oral treatment regimen could prevent an interruption in a patient’s treatment course. Thus, patients with restricted mobility such as the elderly or those who might prefer to remain outside of a hospital or clinic setting for treatment can continue to receive beneficial PI-based treatment. This is especially relevant in the context of the coronavirus disease 2019 pandemic, because many oncology patients might no longer be able to travel to infusion centers for their treatment.
In addition, the effective application of digital technology to record treatment adherence and ePROs can help these patients stay “on track” with their treatment course despite not being able to regularly meet face-to-face with their physician. The digital technology could also have implications for future clinical trial designs. The results from our preliminary analysis support the continued enrollment to the planned 160 patients and further exploration of the benefit of the iCT approach by US region and patient subgroup, including racial and ethnic minorities. The full benefit of the iCT in the US MM-6 patient population will become more defined as the data continue to mature.
Clinical Practice Points
-
•
In MM clinical trials, longer term or continuous PI-based therapy has resulted in prolonged PFS and OS compared with shorter, fixed-duration therapy; however, the outcomes in routine clinical practice have often not matched those obtained in clinical studies, and the median treatment duration for PI-based therapy has often been shorter.
-
•
Furthermore, up to 40% of real-world patients with NDMM might be ineligible for participation in clinical trials because of various factors such as older age and greater comorbidity burden.
-
•
The US MM-6 study was, therefore, designed to evaluate a novel iCT approach in the community-based setting with the aims of increasing PI-based treatment duration and adherence, maintaining HRQoL, and improving outcomes in a representative, real-world community-based population of patients with NDMM.
-
•
The use of this novel iCT approach from parenteral bortezomib-based to oral ixazomib-based therapy facilitated long-term PI-based treatment that is well tolerated in real-world, non-transplant-eligible patients with NDMM.
-
•
The preliminary findings have indicated that the iCT approach results in promising efficacy and high medication adherence, with no adverse impact on patients’ HRQoL or treatment satisfaction.
-
•
The results from the present study have highlighted the feasibility of using mobile and wearable digital devices to collect PROs and actigraphy (activity and sleep) data in the setting of routine clinical practice.
-
•
The use of such digital approaches could have implications for future clinical trial designs.
-
•
The use of iCT and digital technology for ePRO collection enabled continued PI-based treatment for, and monitoring of, patients with restricted mobility who have difficulty traveling to or accessing infusion centers and for those who might prefer to remain outside of a hospital or clinic setting for their therapy.
Disclosures
H.A.Y. has participated on the speakers bureau for Amgen, Takeda, Janssen, AstraZeneca, Karyopharm, and BeiGene and advisory meetings for Takeda, Karyopharm, and Amgen. S.J.N., R.H.F., D.C., B.D., V.L., and P.W. are employed by Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. S.G. has participated in advisory councils or committees for Takeda, Celgene, Genenetech, and Akcea and received honoraria from Takeda and Celgene. C.A.Y. has participated in the speakers bureau for Takeda. K.B. is employed by, and owns stock or shares in, Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. R.E.B. has been a consultant to Alexion; consultant to and received honoraria from AbbVie; consultant to, received honoraria from, and participated in the speakers bureau for, Novartis; consultant to and participated in the speakers bureau for Jansen Bioncology and Pharmacyclics; consultant to, received research funding from, and participated in the speakers bureau for Puma; received research funding and participated in the speakers bureau for Takeda and Incyte; received honoraria from Bayer, Seattle Genetics, Coheris, Kite Pharma, Celgene, and Helsin; received honoraria from and participated in the speakers bureau for Amgen; and participated in the speakers bureau for Pfizer, BMS, Tessaro, AstraZeneca, Genomic Health, Sanofi Oncology, Clovis Oncology, Exelexis, and Lilly. R.B. has received honoraria for participation in the speakers bureau for Takeda. R.M.R. is employed by Rocky Mountain Cancer Centers, LLC; has been a member of an advisory council or committee; and has received consulting fees from BMS/Celgene, Takeda, and Amgen/Onyx. The remaining authors have stated that they have no conflicts of interest.
Acknowledgments
The present study was funded by Millennium Pharmaceuticals, Inc, a wholly owned subsidiary of Takeda Pharmaceutical Company (Cambridge, MA). Representatives of the sponsor, included in the author list, are responsible for the study design, collection, analysis, and interpretation of data, writing the report, and the decision to submit for publication. The authors thank all the patients and their families and all investigators for their valuable contributions to the present study. The authors also acknowledge Matthew Cantor and Peter Kelly from Koneksa for actigraphy data collection and analysis and Jenny Wilkinson of FireKite, an Ashfield company, part of UDG Healthcare Plc, for providing professional medical writing support, which was funded by Millennium Pharmaceuticals, Inc, Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, and complied with the Good Publication Practice-3 (GPP3) guidelines (Battisti WP, Wager E, Baltzer L, et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med 2015; 163:461-4). The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants data supporting the results reported in this article, will be made available within three months from initial request, to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection and requirements for consent and anonymization.
Footnotes
Supplemental Tables and Appendix accompanying this article can be found in the online version at https://doi.org/10.1016/j.clml.2020.06.024.
Supplemental Appendix 1. Patients and Methods
Summary of Key Patient Eligibility Criteria
-
•Inclusion criteria
-
○Age ≥ 18 years
-
○Diagnosis of MM using International Myeloma Working Group criteria
-
○Patients must have received 1 previous line of therapy (ie, patients must have completed 3 cycles of bortezomib-based induction in accordance with the regimens listed in the US National Comprehensive Cancer Network guidelines) and have no evidence of PD
-
○Patients with light chain- and free light chain-only disease can be enrolled if they meet all the criteria for a diagnosis of MM
-
○Patients must be considered by their physician to be eligible to receive the IRd (ixazomib, lenalidomide, dexamethasone) regimen
-
○Patients must be transplant-ineligible as determined by their physician or, if transplant-eligible, not expect to undergo transplantation for ≥ 24 months after study enrollment
-
○Eastern Cooperative Oncology Group and/or other performance status of 0 to 2
-
○At enrollment, patients must meet the clinical laboratory criteria in accordance with the ixazomib prescribing information
-
○
-
•Exclusion criteria
-
○Participation in other interventional clinical trials within 30 days of the start of the US MM-6 trial and throughout the duration of the US MM-6 trial
-
○Female patients who are lactating or have a positive serum pregnancy test during the screening period
-
○Failure to have fully recovered (ie, grade ≤ 1 toxicity) from the reversible effects of previous chemotherapy
-
○Major surgery within 14 days before enrollment
-
○Radiotherapy within 14 days before enrollment
-
○Central nervous system involvement
-
○Infection requiring systemic antibiotic therapy or other serious infection within 14 days before study enrollment
-
○Evidence of current uncontrolled cardiovascular conditions, including uncontrolled hypertension, uncontrolled cardiac arrhythmias, symptomatic congestive heart failure, unstable angina, or myocardial infarction within the previous 6 months
-
○Systemic treatment within 14 days before the first dose of ixazomib with strong CYP3A inducers (ie, rifampin, rifapentine, rifabutin, carbamazepine, phenytoin, phenobarbital) or the use of Ginkgo biloba or St John’s wort
-
○Ongoing or active systemic infection, active hepatitis B or C virus infection, or known human immunodeficiency virus positivity
-
○Any serious medical or psychiatric illness that could, in the investigator’s opinion, potentially interfere with the completion of treatment according to the study protocol
-
○Known allergy to any of the study medications, their analogs, or excipients in the various formulations of any agent
-
○Known gastrointestinal disease or gastrointestinal procedure that could interfere with the oral absorption or tolerance of ixazomib, including difficulty swallowing
-
○Diagnosed or treated for another malignancy within 2 years before study enrollment or previously diagnosed with another malignancy and any evidence of residual disease (patients with non-melanoma skin cancer or carcinoma in situ of any type are not excluded if they have undergone complete resection)
-
○The presence of grade ≥ 2 PN or grade 1 PN with pain on clinical examination at enrollment
-
○Previous treatment with ixazomib or previous participation in a study with ixazomib, whether treated with ixazomib or not
-
○PD during first-line therapy
-
○
Summary of Protocol Changes Affecting Study Conduct.
-
•
Patients must have completed 3 cycles of any bortezomib-based induction regimen (previously only VRD [bortezomib, lenalidomide, dexamethasone] or CVD [cyclophosphamide, bortezomib, dexamethasone]). This change was implemented to broaden patient eligibility.
-
•
Patients can receive treatment with IRd for an additional 13 cycles (ie, a total of 39 cycles). The treatment period was extended because some patients had already reached the previous study maximum of 26 cycles of IRd without disease progression or discontinuation. The extension, therefore, allows for the opportunity to collect up to an additional year’s worth of data.
-
•
Six additional sites were included to capture more minority, social, and economic parameters throughout the US community.
-
•
The key secondary endpoint of the “duration of complete response (CR)” was modified to the “duration of response.”
-
•
The time to subsequent MM treatment and/or transplantation was added as a secondary endpoint.
-
•
The definition of PFS was updated such that the primary analysis for 2-year PFS will be conducted 1 year after the last patient has been enrolled.
Endpoint Definitions
The proportions of patients achieving a PR, VGPR, and CR were determined. The duration of response was defined as the time from the date of the first documentation of a PR or better to the date of the first documentation of PD for responding patients. The duration of PI therapy, including bortezomib-based induction, was defined as the time from the date of the first administration of the bortezomib-based induction regimen to the date of the last administration of ixazomib, lenalidomide, or dexamethasone. The duration of IRd was defined as the time from the date of the first administration of IRd to the date of the last administration of ixazomib, lenalidomide, or dexamethasone. The duration of ixazomib therapy was defined as the time from the date of the first administration of ixazomib to the date of the last administration of ixazomib. The relative dose intensity for each study drug was defined as 100 × (total amount of dose taken)/(total prescribed dose of treated cycles), where the total prescribed dose equaled (dose prescribed at enrollment × number of prescribed doses per cycle × the number of treated cycles).
Scoring for HRQoL and Treatment Satisfaction Questionnaires
The EORTC QLQ-C30 global health status/QoL scale has a range of 0 to 100, for which positive and negative changes indicate improvement and deterioration, respectively, in QoL. The global health status/QoL is derived from items 29 and 30 of the EORTC QLQ-C30 (version 3). The EORTC QoL questionnaire-MM module (QLQ-MY20) item 43 (“Did you have tingling hands or feet?”) measures the burden of PN symptoms using the following scores: 1, not at all; 2, a little; 3, quite a bit; and 4, very much, with higher scores indicating an increase in symptoms. The TSQM-9 subscales (effectiveness, convenience, and global satisfaction) have a range of 0 to 100. Positive and negative changes indicate an increase and decrease, respectively, in treatment satisfaction.
Statistical Analysis
The data are summarized descriptively. The 95% confidence intervals for the response rates were calculated using the exact binomial method. The 12-month PFS rate was estimated using the Kaplan-Meier method.
For the ePROs, the actual values and changes from cycle 1 (ePRO baseline; assessed at the end of cycle 1) over time during IRd treatment were determined in the intent-to-treat population among patients with data at ePRO baseline and at ≥ 1 post-ePRO baseline IRd cycle for the global health status/QoL subscale score (derived from items 29 and 30 in the EORTC QLQ-C30); PN from item 43 of the EORTC QLQ-MY20; and 3 TSQM-9 subscale scores (effectiveness, convenience, and global satisfaction). Furthermore, considering variation in the timing of the ePRO data captured during each IRd cycle (eg, sites could have launched the ePROs off schedule), only those ePRO assessments completed between day 22 of each cycle and day 3 of the subsequent cycle were used for the ePRO analyses. Thus, the initial ePRO assessments used in these analyses (ie, ePRO baseline assessments) occurred at the end of cycle 1. In future examinations of these study data, we plan to explore analysis of clinically relevant ePRO data that might have been captured beyond the day 22 to day 3 cycle window.
ePRO completion proportions for each ePRO were estimated by dividing the number of questionnaires that the study participants completed (ie, the entire, not partial, questionnaire had been completed) by the total number of questionnaires that had been launched to the study participants during the study period. This calculation did not account for cases in which a questionnaire was not launched by the site to the patient.
For the actigraphy data collection to be compliant, the actigraph unit had to be worn for ≥ 14 days per treatment cycle, although these days did not need to be consecutive. A compliant day was one during which the device was worn for ≥ 12 hours (ie, the patient had been recorded as moving in ≥ 12 of the hours of that day). Days for which actigraphy data were available but during which the patient had not been moving in ≥ 12 of the hours of that day were considered noncompliant and were not included in the analyses. For actigraphy data analysis, outliers (defined as > 4 SDs from the mean) were removed; the mean and SD were then recomputed until no outliers remained. Sleep time refers to all periods in which the actigraph unit reported deep sleep, light sleep, or awake time as a part of the sleep record (days with no recorded sleep period were excluded from the sleep time analyses).
Results
For the ePRO data, the completion proportion for EORTC QLQ-C30 was calculated for 49 patients: 469 of 482 questionnaires had been completed (97.30%). The completion proportion for EORTC QLQ-MY20 was calculated for 50 patients: 480 of 492 questionnaires had been completed (97.56%). The completion proportion for TSQM-9 was calculated for 50 patients: 462 of 468 questionnaires had been completed (98.72%). The completion proportion for the monthly medication adherence questionnaire was calculated for 47 patients: 445 of 457 questionnaires had been completed (97.37%). These completion proportions were estimated among the patients who had been included in the ePRO analyses (ie, among patients with data at ePRO baseline and at ≥ 1 post-ePRO baseline IRd cycle, captured within the day 22 to day 3 cycle windows).
Supplemental Table 1.
Baseline Demographics and Disease Characteristics by Agea (n = 84)
| Characteristic | Age Group, y |
|
|---|---|---|
| <75 (n = 47) | ≥75 (n = 37) | |
| Age, y | ||
| Median | 68 | 78 |
| Range | 49-74 | 75-90 |
| Age ≥ 65 y, n (%) | 30 (64) | 37 (100) |
| Male gender, n (%) | 23 (49) | 18 (49) |
| Race, n (%) | ||
| White | 37 (79) | 24 (65) |
| Black/African American | 6 (13) | 7 (19) |
| Asian | 1 (2) | 1 (3) |
| Multiple | 0 (0) | 1 (3) |
| Missing | 3 (6) | 4 (11) |
| Ethnicity, n (%) | ||
| Hispanic/Latino | 6 (13) | 2 (5) |
| Not Hispanic/Latino | 39 (83) | 33 (89) |
| Not reported/unknown | 2 (4) | 2 (5) |
| ISS stage at initial diagnosis, n (%) | ||
| I | 14 (30) | 8 (22) |
| II | 14 (30) | 11 (30) |
| III | 12 (26) | 17 (46) |
| Unknown | 7 (15) | 1 (3) |
| Type of myeloma at initial diagnosis, n (%) | ||
| Heavy chain | ||
| IgG | 29 (62) | 21 (57) |
| IgA | 10 (21) | 6 (16) |
| IgD | 1 (2) | 0 (0) |
| IgM | 0 (0) | 1 (3) |
| Multiple | 3 (6) | 4 (11) |
| Missing | 4 (9) | 5 (14) |
| Light chain | ||
| Kappa | 33 (70) | 18 (49) |
| Lambda | 10 (21) | 17 (46) |
| Multiple | 3 (6) | 2 (5) |
| Missing | 1 (2) | 0 (0) |
| Lytic bone disease, n (%) | 20 (43) | 15 (41) |
| Extramedullary disease, n (%) | 4 (9) | 2 (5) |
| Creatinine clearance,b mL/min | ||
| Median | 81 | 63 |
| Range | 12-226 | 19-133 |
| Calculated creatinine clearance < 30 mL/min, n (%) | 3 (6) | 1 (3) |
Abbreviation: ISS = International Staging System.
At enrollment (or initial diagnosis for ISS stage and type of myeloma).
Patients aged < 75 years totaled 42 and patients aged ≥ 75 years totaled 34.
Supplemental Table 2.
Duration of Therapy by Agea (n = 84)
| Variable | Therapy Duration, mo |
|
|---|---|---|
| Age < 75 y (n = 47) | Age ≥ 75 y (n = 37) | |
| PI therapy, including bortezomib-based inductionb | ||
| Mean ± SD | 10.8 ± 5.6 | 9.3 ± 5.6 |
| Medianc | 9.2 | 6.8 |
| Range | 3.0-22.6 | 2.6-26.4 |
| IRdd | ||
| Mean ± SD | 8.0 ± 5.7 | 6.4 ± 5.6 |
| Medianc | 6.3 | 3.9 |
| Range | 0.03-19.9 | 0.03-23.0 |
| Ixazomibe | ||
| Mean ± SD | 7.8 ± 5.7 | 6.0 ± 5.7 |
| Medianc | 6.1 | 3.7 |
| Range | 0.03-19.8 | 0.03-22.8 |
Abbreviations: IRd = ixazomib, lenalidomide, dexamethasone; PI = proteasome inhibitor; SD = standard deviation.
Patients had to continue receiving ixazomib to remain in the study; a dose interruption of ixazomib lasting > 3 weeks or an interruption at the principal investigator’s discretion was considered discontinuation from ixazomib treatment and the patient was discontinued from the study, although they could be followed up for progression-free and overall survival. In addition, at the discretion of the treating physician, patients could discontinue treatment from lenalidomide and/or dexamethasone but continue ixazomib treatment and remain in the study.
Time from the date of the first administration of the bortezomib-based induction regimen to the date of the last administration of ixazomib, lenalidomide, or dexamethasone.
Simple median (ie, not determined using Kaplan-Meier method).
Time from the date of the first administration of IRd to the date of the last administration of ixazomib, lenalidomide, or dexamethasone.
Time from the date of the first administration of ixazomib to the date of the last administration of ixazomib.
Supplemental Table 3.
Investigator-assessed Best Overall Responses After Bortezomib-based Induction and After in-Class Transition to IRd by Age (n = 84)
| Response | Age < 75 y (n = 47) |
Age ≥ 75 y (n = 37) |
||
|---|---|---|---|---|
| Bortezomib-based induction | iCT to IRd | Bortezomib-based induction | iCT to IRd | |
| ORRa | 27 (57) [42-72] | 32 (68) [53-81] | 25 (68) [50-82] | 27 (73) [56-86] |
| ≥VGPR | 11 (23) | 26 (55) | 13 (35) | 20 (54) |
| CRb | 1 (2) [0.1-11] | 14 (30) [17-45] | 2 (5) [0.7-18] | 8 (22) [10-38] |
| VGPR | 10 (21) [11-36] | 12 (26) [14-40] | 11 (30) [16-47] | 12 (32) [18-50] |
| PR | 16 (34) [21-49] | 6 (13) [5-26] | 12 (32) [18-50] | 7 (19) [8-35] |
Data presented as n (%) [95% confidence interval].
Abbreviations: CR = complete response; iCT = in-class transition; IRd = ixazomib, lenalidomide, dexamethasone; ORR = overall response rate; PR = partial response; VGPR = very good partial response.
ORR includes CR, VGPR, plus PR.
CR category includes patients who achieved CR, stringent CR, immunophenotypic CR, and molecular CR.
Supplemental Table 4.
Overview of Safety Profile of IRd by Age (n =84)
| TEAEa | Age, y |
|
|---|---|---|
| <75 (n = 47) | ≥75 (n = 37) | |
| Any-grade TEAE | 44 (94) | 33 (89) |
| Any-grade treatment-related TEAE | 36 (77) | 23 (62) |
| Grade ≥ 3 TEAE | 21 (45) | 19 (51) |
| Grade ≥ 3 treatment-related TEAE | 9 (19) | 12 (32) |
| TEAE leading to drug modificationb | 25 (53) | 17 (46) |
| TEAE leading to drug discontinuationb | 2 (4) | 4 (11) |
| Serious TEAE | 15 (32) | 15 (41) |
| Treatment-related serious TEAE | 4 (9) | 5 (14) |
| On-study deaths | 1 (2) | 1 (3) |
Data presented as n (%).
Abbreviations: IRd = ixazomib, lenalidomide, dexamethasone; TEAE = treatment-emergent adverse event.
TEAEs were defined as adverse events that occurred after administration of the first dose through 30 days after the last dose of the study drug regimen (IRd) TEAEs were considered serious if they resulted in death, were life-threatening, had required inpatient hospitalization or prolongation of an existing hospitalization (excluding planned hospital admissions and surgical procedures for a preexisting condition unless it had deteriorated unexpectedly during the study period), had resulted in persistent or significant disability or incapacity, or were a congenital anomaly/birth defect or a “medically important event”.
Modification or discontinuation of any of the 3 study drugs (ixazomib, lenalidomide, dexamethasone).
Supplemental Table 5.
Frequently Occurring Treatment-emergent Adverse Events During IRd Treatmenta by Age (n = 84)
| TEAE | Age < 75 y (n = 47) |
Age ≥ 75 y (n = 37) |
||
|---|---|---|---|---|
| Any Grade | Grade 3 | Any Grade | Grade 3 | |
| Diarrhea | 19 (40) | 1 (2) | 15 (41) | 5 (14) |
| PN NECb | 17 (36) | 1 (2) | 8 (22) | 1 (3) |
| Fatigue | 13 (28) | 1 (2) | 7 (19) | 1 (3) |
| Nausea | 13 (28) | 0 (0) | 7 (19) | 2 (5) |
| Back pain | 12 (26) | 1 (2) | 4 (11) | 0 (0) |
| Constipation | 10 (21) | 0 (0) | 4 (11) | 0 (0) |
| Hypokalemia | 7 (15) | 0 (0) | 6 (16) | 2 (5) |
| Cough | 10 (21) | 0 (0) | 3 (8) | 0 (0) |
| Dizziness | 6 (13) | 1 (2) | 6 (16) | 0 (0) |
| Peripheral edema | 8 (17) | 0 (0) | 4 (11) | 0 (0) |
| Anemia | 7 (15) | 2 (4) | 4 (11) | 2 (5) |
| Arthralgia | 7 (15) | 0 (0) | 4 (11) | 0 (0) |
| Hypotension | 6 (13) | 1 (2) | 4 (11) | 1 (3) |
| Rash | 6 (13) | 1 (2) | 4 (11) | 1 (3) |
| Pneumonia | 5 (11) | 3 (6) | 4 (11) | 2 (5) |
| Vomiting | 6 (13) | 1 (2) | 3 (8) | 1 (3) |
| Decreased appetite | 4 (9) | 0 (0) | 4 (11) | 1 (3) |
| Platelet count decreased | 5 (11) | 1 (2) | 3 (8) | 0 (0) |
| Pyrexia | 5 (11) | 0 (0) | 3 (8) | 0 (0) |
| Fall | 5 (11) | 2 (4) | 2 (5) | 0 (0) |
| Hypocalcemia | 5 (11) | 0c (0) | 2 (5) | 0 (0) |
| Muscle weakness | 2 (4) | 0 (0) | 4 (11) | 1 (3) |
| Peripheral swelling | 5 (11) | 0 (0) | 1 (3) | 0 (0) |
| Thrombocytopenia | 6 (13) | 2 (4) | 0 (0) | 0 (0) |
| Dehydration | 1 (2) | 0 (0) | 4 (11) | 2 (5) |
| Syncope | 2 (4) | 2 (4) | 3 (8) | 3 (8) |
Data presented as n (%).
Abbreviations: IRd = ixazomib, lenalidomide, dexamethasone; PN NEC = peripheral neuropathy, not elsewhere classified; TEAE = treatment-emergent adverse event.
Cutoffs for inclusion were ≥ 10% of patients with any grade or ≥ 5% of patients at grade 3 in either subgroup.
High-level term.
One patient in the < 75-year age subgroup had experienced grade 4 hypercalcemia.
References
- 1.Kumar S.K., Callander N.S., Hillengass J., et al. NCCN guidelines insights: multiple myeloma, version 1.2020. J Natl Compr Canc Netw. 2019;17:1154–1165. doi: 10.6004/jnccn.2019.0049. [DOI] [PubMed] [Google Scholar]
- 2.Durie B.G.M., Hoering A., Abidi M.H., et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389:519–527. doi: 10.1016/S0140-6736(16)31594-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.San Miguel J.F., Schlag R., Khuageva N.K., et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 4.San Miguel J.F., Schlag R., Khuageva N.K., et al. Persistent overall survival benefit and no increased risk of second malignancies with bortezomib-melphalan-prednisone versus melphalan-prednisone in patients with previously untreated multiple myeloma. J Clin Oncol. 2013;31:448–455. doi: 10.1200/JCO.2012.41.6180. [DOI] [PubMed] [Google Scholar]
- 5.Mateos M.V., Richardson P.G., Dimopoulos M.A., et al. Effect of cumulative bortezomib dose on survival in multiple myeloma patients receiving bortezomib-melphalan-prednisone in the phase III VISTA study. Am J Hematol. 2015;90:314–319. doi: 10.1002/ajh.23933. [DOI] [PubMed] [Google Scholar]
- 6.Jimenez-Zepeda V.H., Duggan P., Neri P., Tay J., Bahlis N.J. Bortezomib-containing regimens (BCR) for the treatment of non-transplant eligible multiple myeloma. Ann Hematol. 2017;96:431–439. doi: 10.1007/s00277-016-2901-x. [DOI] [PubMed] [Google Scholar]
- 7.Richardson P.G., San Miguel J.F., Moreau P., et al. Interpreting clinical trial data in multiple myeloma: translating findings to the real-world setting. Blood Cancer J. 2018;8:109. doi: 10.1038/s41408-018-0141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah J.J., Abonour R., Gasparetto C., et al. Analysis of common eligibility criteria of randomized controlled trials in newly diagnosed multiple myeloma patients and extrapolating outcomes. Clin Lymphoma Myeloma Leuk. 2017;17:575–583. doi: 10.1016/j.clml.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Chari A., Parikh K., Ni Q., Abouzaid S. Treatment patterns and clinical and economic outcomes in patients with newly diagnosed multiple myeloma treated with lenalidomide- and/or bortezomib-containing regimens without stem cell transplant in a real-world setting. Clin Lymphoma Myeloma Leuk. 2019;19:645–655. doi: 10.1016/j.clml.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Chari A., Richardson P.G., Romanus D., et al. Real-world outcomes and factors impacting treatment choice in relapsed and/or refractory multiple myeloma (RRMM): a comparison of VRd, KRd, and IRd. Expert Rev Hematol. 2020;13:421–433. doi: 10.1080/17474086.2020.1729734. [DOI] [PubMed] [Google Scholar]
- 11.Jagannath S., Roy A., Kish J., et al. Real-world treatment patterns and associated progression-free survival in relapsed/refractory multiple myeloma among US community oncology practices. Expert Rev Hematol. 2016;9:707–717. doi: 10.1080/17474086.2016.1195254. [DOI] [PubMed] [Google Scholar]
- 12.Baz R., Lin H.M., Hui A.M., et al. Development of a conceptual model to illustrate the impact of multiple myeloma and its treatment on health-related quality of life. Support Care Cancer. 2015;23:2789–2797. doi: 10.1007/s00520-015-2644-6. [DOI] [PubMed] [Google Scholar]
- 13.VELCADE® (bortezomib) for injection. Velcade US Prescribing Information 2019. https://www.velcade.com/files/pdfs/VELCADE_PRESCRIBING_INFORMATION.pdf Available at: Accessed: June 17, 2020.
- 14.Fiala M., Dukeman J., Stockerl-Goldstein K., Tomasson M., Wildes T., Vij R. The real-world characteristics and outcomes of newly diagnosed myeloma patients ineligible for clinical trials. Clin Lymphoma Myeloma Leuk. 2017;17:e55–e56. [Google Scholar]
- 15.Hungria V.T.M., Lee H.C., Abonour R., et al. Real-world (RW) multiple myeloma (MM) patients (Pts) remain under-represented in clinical trials based on standard laboratory parameters and baseline characteristics: analysis of over 3,000 Pts from the insight MM global, prospective, observational study. Blood. 2019;134:1887. [Google Scholar]
- 16.Gupta N., Hanley M.J., Xia C., Labotka R., Harvey R.D., Venkatakrishnan K. Clinical pharmacology of ixazomib: the first oral proteasome inhibitor. Clin Pharmacokinet. 2019;58:431–449. doi: 10.1007/s40262-018-0702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NINLARO® (ixazomib) NINLARO US Prescribing Information 2016. https://www.ninlarohcp.com/pdf/prescribing-information.pdf Available at: Accessed: June 17, 2020.
- 18.Hajek R., Terpos E., Lee H.C., et al. Ixazomib plus lenalidomide-dexamethasone (IRd) in relapsed/refractory multiple myeloma (MM) patients (Pts)—effectiveness in routine clinical practice is similar to the efficacy in the phase 3 tourmaline-MM1 trial: a pooled analysis from the insight MM observational study and the Czech registry of monoclonal gammopathies (RMG) Blood. 2018;132:1971. [Google Scholar]
- 19.Hajek R., Minarik J., Straub J., et al. Closing the efficacy and effectiveness gap: outcomes in relapsed/refractory multiple myeloma (RRMM) patients (Pts) treated with ixazomib-lenalidomide-dexamethasone (IRd) in routine clinical practice remain comparable to the outcomes reported in the phase 3 tourmaline-MM1 study. Blood. 2019;134:1845. [Google Scholar]
- 20.Cohen Y.C., Magen H., Lavi N., et al. Ixazomib-based regimens for relapsed/refractory multiple myeloma: are real-world data compatible with clinical trial outcomes? A multi-site Israeli registry study. Ann Hematol. 2020;99:1273–1281. doi: 10.1007/s00277-020-03985-9. [DOI] [PubMed] [Google Scholar]
- 21.Ziff M., Cheesman S., Kyriakou C., et al. Real world use of ixazomib with lenalidomide and dexamethasone for patients with relapsed and relapsed refractory multiple myeloma. Haematologica. 2017;102:786–787. [Google Scholar]
- 22.Minarik J., Krhovska P., Jelinek T., et al. Treatment of relapsed and refractory multiple myeloma with fully oral triplet IRD (ixazomib, lenalidomide and dexamethasone) is safe and with significant therapeutic outcomes. Blood. 2018;132:1959. [Google Scholar]
- 23.Minarik J., Pika T., Radocha J., et al. Overall survival benefit of ixazomib, lenalidomide and dexamethasone (IRD) over lenalidomide and dexamethasone (RD) in RRMM patients treated in routine clinical practice: results from the Czech registry of monoclonal gammopathies (RMG) Blood. 2019;134:3139. [Google Scholar]
- 24.Terpos E., Maouche N., Minarik J., et al. “Real world” data on the efficacy and safety of ixazomib in combination with lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: a combined study from the Greek, Czech and UK databases. Blood. 2017;130:3087. [Google Scholar]
- 25.Moreau P., Masszi T., Grzasko N., et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374:1621–1634. doi: 10.1056/NEJMoa1516282. [DOI] [PubMed] [Google Scholar]
- 26.Rajkumar S.V., Dimopoulos M.A., Palumbo A., et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–e548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 27.Palumbo A., Rajkumar S.V., San Miguel J.F., et al. International Myeloma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol. 2014;32:587–600. doi: 10.1200/JCO.2013.48.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar S.K., Berdeja J.G., Niesvizky R., et al. Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open-label phase 1/2 study. Lancet Oncol. 2014;15:1503–1512. doi: 10.1016/S1470-2045(14)71125-8. [DOI] [PubMed] [Google Scholar]
- 29.Niesvizky R., Flinn I.W., Rifkin R., et al. Community-based phase IIIB trial of three UPFRONT bortezomib-based myeloma regimens. J Clin Oncol. 2015;33:3921–3929. doi: 10.1200/JCO.2014.58.7618. [DOI] [PubMed] [Google Scholar]
- 30.Chari A., Romanus D., Palumbo A., et al. Randomized clinical trial representativeness and outcomes in real-world patients: comparison of 6 hallmark randomized clinical trials of relapsed/refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2020;20:8–17. doi: 10.1016/j.clml.2019.09.625. [DOI] [PubMed] [Google Scholar]
- 31.Kim E.S., Bruinooge S.S., Roberts S., et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research joint research statement. J Clin Oncol. 2017;35:3737–3744. doi: 10.1200/JCO.2017.73.7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar S.K., Buadi F.K., LaPlant B., et al. Phase 1/2 trial of ixazomib, cyclophosphamide and dexamethasone in patients with previously untreated symptomatic multiple myeloma. Blood Cancer J. 2018;8:70. doi: 10.1038/s41408-018-0106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dimopoulos M.A., Grosicki S., Jedrzejczak W.W., et al. All-oral ixazomib, cyclophosphamide, and dexamethasone for transplant-ineligible patients with newly diagnosed multiple myeloma. Eur J Cancer. 2019;106:89–98. doi: 10.1016/j.ejca.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Kumar S., Moreau P., Hari P., et al. Management of adverse events associated with ixazomib plus lenalidomide/dexamethasone in relapsed/refractory multiple myeloma. Br J Haematol. 2017;178:571–582. doi: 10.1111/bjh.14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tudor-Locke C., Craig C.L., Aoyagi Y., et al. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. 2011;8:80. doi: 10.1186/1479-5868-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell S.S., Murphy P.J. The nature of spontaneous sleep across adulthood. J Sleep Res. 2007;16:24–32. doi: 10.1111/j.1365-2869.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- 37.Coleman E.A., Goodwin J.A., Coon S.K., et al. Fatigue, sleep, pain, mood, and performance status in patients with multiple myeloma. Cancer Nurs. 2011;34:219–227. doi: 10.1097/NCC.0b013e3181f9904d. [DOI] [PMC free article] [PubMed] [Google Scholar]