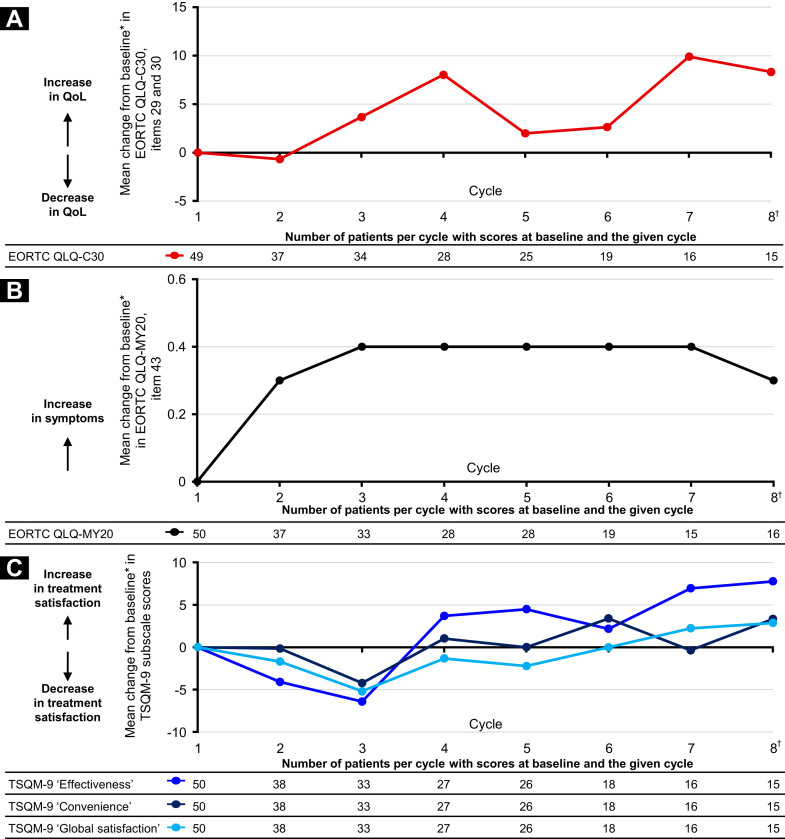
Figure 3.
Electronic Patient-Reported Outcomes (ePROs) During Treatment With IRd (Ixazomib, Lenalidomide, Dexamethasone). (A) Mean Change From ePRO Baseline in European Organization for the Research and Treatment of Cancer (EORTC) Core Quality of Life (QoL) Questionnaire (QLQ-C30) Global Health Status/QoL Subscale Score (Items 29 and 30). (B) Mean Change From ePRO Baseline in EORTC QoL Questionnaire–Multiple Myeloma Module (QLQ-MY20) Item 43 (Peripheral Neuropathy). (C) Mean Change From ePRO Baseline in Treatment Satisfaction Questionnaire for Medication (TSQM)-9 Subscale Scores. ∗ePRO Baseline Was Defined as Reported Measurement at End of Cycle 1 of IRd; Change From ePRO Baseline Was Only Calculated at Post-ePRO Baseline IRd Cycles for Which a Value Was Present and for Patients With an ePRO Baseline Value. †Data Were Available for up to 24 Cycles for Each Measure. Data Not Shown After Cycle 8 Because of Small Patient Numbers (n ≤ 10). Global Health Status/QoL Subscale Was Derived From Items 29 and 30 of the EORTC QLQ-C30 (Version 3). This Scale Has a Range of 0 to 100. Positive and Negative Changes Indicate an Improvement and Deterioration, Respectively, in QoL. EORTC QLQ-MY20 Item 43 (“Did You Have Tingling Hands or Feet?”) Measured the Burden of Peripheral Neuropathy Symptoms Using a Score Range of 1 to 4 (1, Not at All; 2, a Little; 3, Quite a Bit; and 4, Very Much). A Higher Score Indicates an Increase in Symptoms. The TSQM-9 Subscales (Effectiveness, Convenience, and Global Satisfaction) Have a Range of 0 to 100. Positive and Negative Changes Indicate an Increase and Decrease, Respectively, in Treatment Satisfaction