Abstract
Objective:
The objective of the study is to compare the efficacy of Thoracic epidural with Intercostal block plus intravenous morphine infusion for postoperative analgesia in patients undergoing elective thoracotomy.
Methodology and Design:
This study is designed as a prospective randomized clinical trial.
Setting:
Christian Medical College Hospital, Vellore, India.
Participants:
Patients undergoing elective thoracic surgery through posterolateral thoracotomy.
Intervention:
In Group A (TEA) patients epidural catheter was inserted at T5-6 level before induction of GA and analgesia was activated using 0.25% of bupivacaine towards the end of the surgery, before chest closure and infusion of 0.1% bupivacaine with 2 mcg/ml of fentanyl was started. In Group B (ICN) patients, an intercostal blockade of the 5 intercostal spaces was performed by the surgeon just before chest closure using 0.25% bupivacaine and a continuous intravenous morphine infusion of 0.015-0.02 mg/kg/hr was started.
Measurements:
Assessment of resting and dynamic pain intensity using Numerical rating scale and sedation using Ramsay sedation scale was done and recorded at 1, 6,12,18,24 hours during the first postoperative day. The other parameters that were measured include side effects and the requirement of rescue analgesia. Results: Resting and Dynamic (NRS) pain scores were less in Group A (TEA) than Group B (ICN). In the first 12 hours, the differences in both the resting (P = 0.0505) and dynamic (P = 0.0307) pain scores were statistically significant. By the end of the first postoperative day, sedation scores were more or less similar in both groups. The incidence of side effects and requirement of rescue analgesia were found to be similar in both the groups.
Conclusion:
To summarize, though the results show a slightly better quality of analgesia with the thoracic epidural, the difference being clinically insignificant intercostal blockade could be considered as a valid alternative.
Keywords: Bupivacaine, fentanyl, intercostal block, morphine, Numerical Rating Scale, thoracic epidural analgesia, thoracotomy
Background
Thoracotomy is considered to be one of the most painful surgical procedures.[1] The effective treatment of acute postthoracotomy pain is important to keep the patient comfortable and to minimize pulmonary complications. Early and aggressive treatment of acute postoperative pain may help to reduce the high incidence of chronic pain.[2,3] Various analgesic management strategies have evolved, often the regional analgesic techniques being favored over the systemic administration of opioids. Thoracic epidural analgesia (TEA) has been the most widely used form of postthoracotomy pain relief. In spite of the good analgesic profile, TEA has been associated with complications, and hence, alternative regional techniques for postthoracotomy pain control have been sought for.[4] Based on our institutional experience, a combination of intercostal nerve blocks (ICNs) performed by the surgeon under direct vision before closure of the thoracotomy and intravenous infusion with morphine, appeared to be a satisfactory alternative to a thoracic epidural with fentanyl and bupivacaine for postthoracotomy analgesia. Thus, the objectives of the present study were to compare TEA with ICN block with intravenous morphine in terms of quality of analgesia, and the incidence of adverse effects in patients undergoing thoracic surgery through a posterolateral thoracotomy.
Methods
After institutional review board approval and written informed consent, 50 adults (18 years and older), American Society of Anesthesiologists (ASA) I, II, or III patients scheduled for surgery through a posterolateral thoracotomy in whom no contraindications for thoracic epidural or intercostal blockade, were recruited in the study. Patients were assigned to one of the two groups by computer-generated randomized numbers using block randomization technique: Group A: TEA and Group B: intercostal block (ICN with bupivacaine and continuous infusion of morphine. Patient information sheet was given in the thoracic outpatient department to those fulfilling the criteria.
All the patients were evaluated by the principal investigator, who explained the procedure and the study was explained and informed consent was obtained. The patients were familiarized with the use of Numerical Rating scale (NRS). Consented patients were assigned to either TEA group or ICN group by a computer-generated random allocation. In Group A (TEA) patients, thoracic epidural was cited in sitting position at T5-6 level and catheter introduced 5 cm into the epidural space. Loss-of-resistance technique with saline was used to identify the epidural space. A test dose of 3 ml of 0.5% bupivacaine was given and response to cold stimulation was used to evaluate adequacy of epidural blockade. Anesthesia techniques were standardized; after preoxygenation with 100% oxygen for 3 min, anesthesia was induced with propofol 1–2 mg/kg, vecuronium 0.1 mg/kg, and 2 mcg/kg of fentanyl. Additional doses of fentanyl up to a maximum total dose of 5 mcg/kg and 0.1 mg/kg of morphine were used during surgery. Any adverse hemodynamic events such as hypotension and tachycardia were appropriately treated. Intubation done with appropriate double-lumen tube and position was confirmed with FOB. Anesthesia was maintained with 1 minimal alveolar concentration isoflurane. All the vital parameters, drugs administered, any adverse hemodynamic events, and total amount of intravenous fluids given were noted in the data collection sheet.
In Group A (TEA) patients, epidural analgesia was activated using 5–10 ml bolus of 0.25% of bupivacaine which was administered over a period of 20 min toward the end of the surgery, before chest closure and infusion of 0.1% bupivacaine with 2 mcg/ml of fentanyl was started at a rate of 5–8 ml/h through syringe pump (Terumo Model). In Group B (ICN) patients, an intercostal blockade of the five intercostal spaces (3rd to 8th intercostal spaces) and infiltration of the chest drain exit site were performed by the surgeon under direct vision just before closure of the thoracotomy using a total of 20 ml of plain 0.25% bupivacaine. A continuous intravenous morphine infusion of 0.015–0.02 mg/kg/h was started using an elastomeric device (DOSI-FUSER®) for 48 h.
After completion of surgery, all patients were extubated and shifted to thoracic high-dependency unit (HDU). Standard postoperative care and monitoring was done for all patients in thoracic HDU for 48 h. During the first postoperative day, as per the thoracic HDU protocol, all the patients received paracetamol, 1 g, intravenously every 8 h, and oxygen, 5 L/min, during the first 6 h. Vital parameters such as heart rate, blood pressure, and respiratory rate were noted every hour. Pain was assessed using the Numerical Rating Scale (NRS) every 2 h by the principal investigator. Sedation scale was also noted according to the Ramsay Sedation Scale. However, the highest pain and sedation scores during every 6 h period (1, 6, 12, 18, and 24 h) were documented for the statistical analysis.
Rescue analgesia was given with injection morphine 2 mg if the patient complained of pain and NRS >5 depending on the HDU protocol. Dose, time (hours after surgery), and number of times of rescue analgesia had been administered were noted. Adverse events such as nausea, vomiting, pruritis, hypotension, and respiratory depression were noted and treated accordingly. Bromage motor blockade scale was monitored in the TEA group. Physiotherapy and incentive spirometry were given to all patients and pain scores were recorded while performing breathing exercises. Patients were ambulated on the morning of the 2nd postoperative day. The patients were followed up for 48 h.
Based on a previously published study, taking postoperative pain assessed by Numerical Rating Scale as the primary objective, standard deviation obtained was 1.5 and expected difference between the means was calculated as 1.2. Assuming the power of our study 80% and an alpha error of 0.05, the sample size was calculated to be 50.[5]
The data collected from the patients were entered in Epidata. Pain scores at different time points of 1, 6, 12, 18, and 24 h during the 1st postoperative day between the two groups were compared using Mann–Whitney U-test. The statistical software used was STATA 11 (Strata Corp., College Station, Texas, USA). Results were considered statistically significant if P < 0.05.
Results
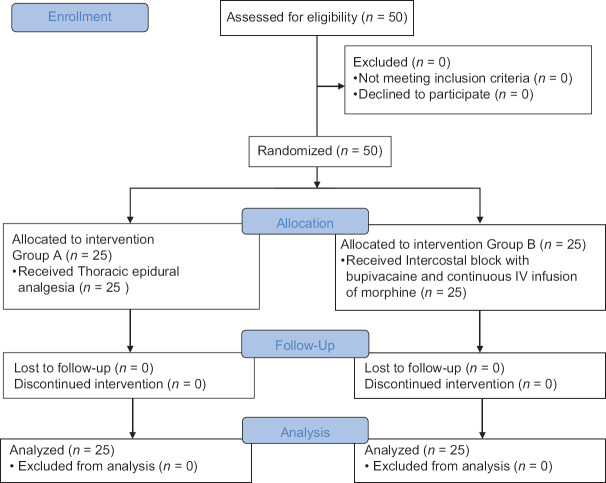
The flow of patients through the trial is described in Figure 1. Twenty-five patients in each group were studied. Both the groups were similar in age, sex, height, weight, ASA classification [Table 1], and the surgical procedure [Table 2] performed.
Figure 1.
Consort flow diagram
Table 1.
Baseline characteristics
| Patient characteristics | Group A (TEA) n=25 |
Group B (ICN) n=25 |
|---|---|---|
| Mean age (years) | 37.72 | 39 |
| Sex | ||
| Male | 16 | 16 |
| Female | 9 | 9 |
| Mean weight (kg) | 58.96 | 56.4 |
| Mean height (cm) | 158.72 | 157.52 |
| Body mass index (kg/m2) | 23.204 | 22.784 |
| ASA physical status classification | ||
| I | 11 | 9 |
| II | 13 | 15 |
| III | 1 | 1 |
TEA: Thoracic epidural analgesia, ASA: American Society of Anesthesiologists, ICN: Intercostal nerve block
Table 2.
Type of surgery and opioid requirement
| Group A (TEA) n=25 | Group B (ICN) n=25 | |
|---|---|---|
| Type of surgery | ||
| Pneumonectomy | 5 | 2 |
| Lobectomy | 8 | 7 |
| Tumor/cyst excision | 10 | 5 |
| Decortication | 2 | 11 |
| Opioid requirement | ||
| Total dose of fentanyl (µg) | 168.6 (100-200) | 188.6 (150-200) |
| Total dose of morphine (mg) | 6.84 (6-8) | 7.68 (6-10) |
TEA: Thoracic epidural analgesia, ICN: Intercostal nerve block
Intraoperative morphine requirement was 6.84 mg in Group A and 7.68 mg in Group B, whereas the fentanyl requirements were 168.6 μg and 188.6 μg [Table 2], respectively.
There was not much difference among the mean amount of fluids used in both groups (Group A: 1.364 L and Group B: 1.312 L) and was not statistically significant (P = 0.382).
There was a significant difference in the level of sedation [Figure 2], patients in Group B were more sedated than in Group A. In Group A, there was a linear trend in the level of sedation and the mean Ramsay sedation score was around 2, implying patients were cooperative, calm, and oriented. In Group B, there was a declining trend for the first 12 h. During the 1st h, sedation score in Group B was higher (mean 4; P = 0.088), which could be interpreted due to the residual effects of narcotics used intraoperatively. By the end of the 1st postoperative day, sedation scores were more or less similar in both groups.
Figure 2.
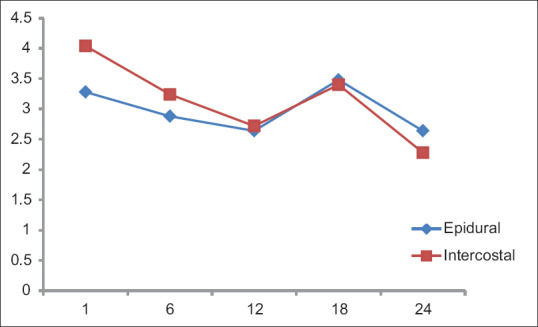
Sedation scale. X-axis: Time (h), Y-axis: Mean
Numerical Rating Scale (NRS) was used for assessing pain intensity and the adequacy of analgesia between the two groups which is rated by the patient themselves so that we can slightly reduce the observer bias, still the patient bias existed. Pain scores were analyzed at different time durations (1, 6, 12, 18, and 24 h) during the 1st postoperative day since pain during the 1st postoperative day is considered very severe and aggressive treatment of acute pain have long-term outcome in thoracic surgery [Tables 3 and 4].
Table 3.
Resting pain score (Numerical Rating Pain Scale) during the 1st postoperative day
| Time frame (h) | Group (n=25) | Mean | SD | P |
|---|---|---|---|---|
| 1st | A (TEA) | 2.8 | 1.55 | 0.0278 |
| B (ICN) | 3.6 | 0.91 | ||
| 6th | A (TEA) | 2.44 | 1.26 | 0.0505 |
| B (ICN) | 3.24 | 1.45 | ||
| 12th | A (TEA) | 2.16 | 1.21 | 0.9918 |
| B (ICN) | 2.08 | 0.95 | ||
| 18th | A (TEA) | 2.08 | 0.75 | 0.0330 |
| B (ICN) | 2.6 | 1.08 | ||
| 24th | A (TEA) | 2 | 1 | 0.1090 |
| B (ICN) | 2.32 | 0.74 |
TEA: Thoracic epidural analgesia, ICN: Intercostal nerve block, SD: Standard deviation
Table 4.
Dynamic pain score (Numerical Rating Pain Scale) during the 1st postoperative day
| Time frame (h) | Group (n=25) | Mean | SD | P |
|---|---|---|---|---|
| 1st | A (TEA) | 2.96 | 1.485 | 0.0105 |
| B (ICN) | 3.96 | 1.019 | ||
| 6th | A (TEA) | 3.16 | 1.462 | 0.0327 |
| B (ICN) | 4.12 | 1.763 | ||
| 12th | A (TEA) | 2.68 | 1.573 | 0.5129 |
| B (ICN) | 2.72 | 1.1 | ||
| 18th | A (TEA) | 3 | 1.322 | 0.1029 |
| B (ICN) | 3.4 | 1.190 | ||
| 24th | A (TEA) | 2.6 | 0.866 | 0.1642 |
| B (ICN) | 2.92 | 0.909 |
TEA: Thoracic epidural analgesia, ICN: Intercostal nerve block, SD: Standard deviation
Resting and dynamic (NRS) pain scores [Figures 3 and 4] were less in Group A than Group B. In the first 12 h, the differences in both the resting (P = 0.0505) and dynamic (P = 0.0307) pain scores were statistically significant. Even though the pain scores at the end of 24 h were lesser in Group A, it was not statistically significant. A steady pain relief was observed in Group A during both rest and exercise. Importantly, the pain scores were never severe in both groups. The highest pain score was observed with Group B, resting score of 3.6 at 1st h after surgery and dynamic score of 4 at 6th h after surgery.
Figure 3.
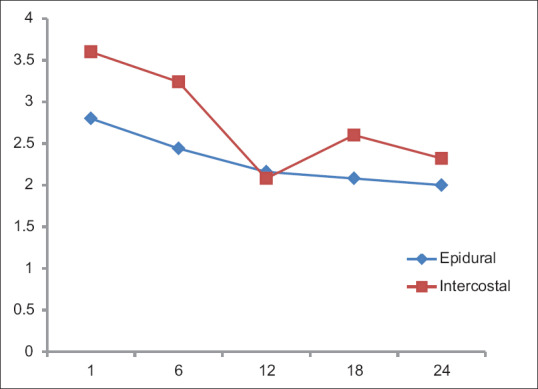
Resting (NRS) pain scale. X-axis: Time (h); Y-axis: Mean NRS
Figure 4.
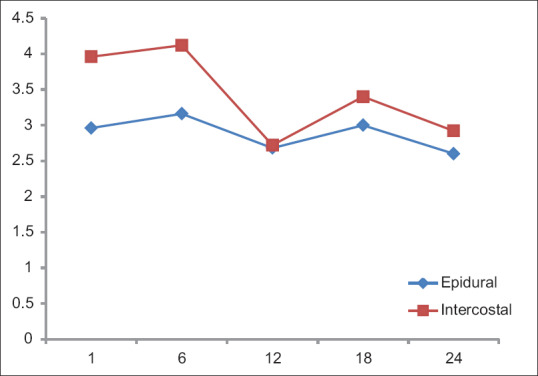
Dynamic (NRS) pain scale. X-axis: Time (h), Y-axis: Mean NRS
With regard to the rescue analgesia [Table 5], almost equal number of patients in both the groups received rescue analgesia. In Group B, five patients received rescue analgesia twice and one patient received thrice. The mean time to receive the first dose of rescue analgesia after surgery was 5.2 h in Group A and 3.9 h in Group B. However, the statistical results are not significant.
Table 5.
Rescue analgesia
| Number of patients requiring rescue analgesia | Number of times rescue analgesia needed | Time for first rescue analgesia | ||||
|---|---|---|---|---|---|---|
| Once | Twice | Thrice | Mean (h) | SD | ||
| A (TEA) n=25 | 11 | 8 | 3 | 0 | 5.272 | 3.663 |
| B (ICN) n=25 | 12 | 7 | 5 | 1 | 3.909 | 3.700 |
| P | 0.777 | 0.494 | 0.2340 | |||
TEA: Thoracic epidural analgesia, ICN: Intercostal nerve block, SD: Standard deviation
No significant differences in the incidence of nausea and vomiting [Figure 5] between Group A and Group B were found. Four patients in epidural group and one patient in the intercostal group had hypotension which was not statistically significant.
Figure 5.
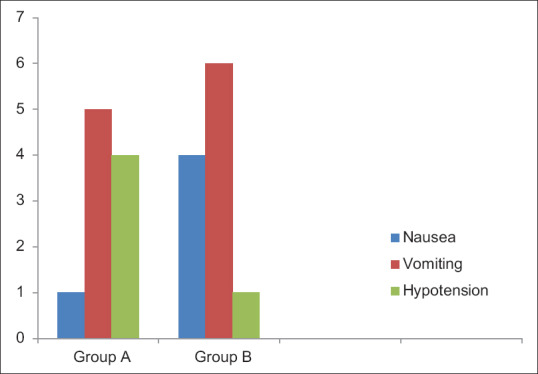
Side effects
None of the patients in either group had respiratory depression or desaturation requiring reintubation.
Modified Bromage scale was used to assess the motor blockade in the epidural Group A. None of the patients in our study had significant motor blockade. Since all patients were catheterized intraoperatively during the surgery as per surgical protocol, the incidence of urinary retention could not be studied.
Discussion
The optimal method of pain relief after thoracotomy is controversial. Improved pain management increases patient comfort, but it is still unclear if postoperative epidural analgesia reduces morbidity or mortality. Thoracic epidural is currently considered the gold standard in many centers for postthoracotomy pain control. However, it is not applicable to all thoracotomy patients and can be associated with side effects, such as hypotension, urinary retention, bradycardia, itching, dural perforation, and in rare cases, may result in epidural hematoma or epidural abscess.[5]
An alternative technique, with similar analgesic effect and without the potential risks, appears as an attractive possibility.[6]
In this study, we compared the two frequently used techniques in our institution (thoracic epidurals and intercostal blocks) for postthoracotomy analgesia in terms of analgesia efficacy and complications. The main findings of our study were the slightly lower resting and dynamic NRS scores in the epidural group and absence of side effects in both the groups.
The resting and dynamic NRS pain scores in both the groups were <4 which has been considered as acceptable control of pain. These results were similar to a study which showed that ICN plus intravenous patient-controlled analgesia morphine is a good alternative for postthoracotomy pain management.[7] In both the groups, some patients experienced breakthrough pain which was treated with rescue analgesia. However, there was no statistical difference in the time to administer the first rescue analgesia and the number of times of administration between the two groups. In our study, in the intercostal group (B), we used morphine at 0.015–0.02 mg/kg/h as a continuous infusion which was delivered using a secure elastomeric device (DOSI-FUSER®) for 48 h to avoid accidental overdosage and the low dose of morphine infusion used helped to reduce the incidence of side effects.
It has been doubted whether a single intercostal block (ICB) could provide effective analgesia, a study[8] has shown that segmental analgesia after a single ICN with bupivacaine could last up to 20 h but required intravenous morphine as a supplement. These results tend to confirm this longer effect or, alternatively, could be explained by the blockade of 5 segments and the routine addition of nonsteroidal anti-inflammatory drugs,[9,10] which have been shown to improve the quality of analgesia and to decrease morphine requirements. The analgesic efficacy and more prolonged effect of the ICB might be obtained with the addition of epinephrine and with the use of liposomal bupivacaine.[11,12]
Intercostal catheter analgesia has shown to lower postoperative visual analog scale scores and reduced opioid requirement as compared to intercostal nerve blockade.[13] However, in our study, we compared the TEA with ICN plus intravenous morphine which is practiced in our institution.
Postoperative sedation is an important and common side effect of morphine infusion, and it has been validated that sedation scale is also an important indicator of impending respiratory depression.[14]
There is a common assumption that adequate pain relief induces sleep. It has been shown that clinically induced sedation with opioids does not assure adequate pain relief.[15] In our study, the sedation scores were significantly different between the two groups. During the 1st h after surgery patients in the intercostal group were more sedated, the difference might be due to the effects of general anesthesia and drugs used intraoperatively. However, at the end of the 1st day, mean sedation scores and pain scores were similar in both the groups which could be due to the accumulation of morphine after continuous infusion and achieving its effective therapeutic concentration.
Many of the unwanted side effects of epidural analgesia techniques are secondary to the associated bilateral sympathetic, sensory, and motor blockade or the addition of opioids to the local anesthetic solution.[16] Both hypotension and urinary retention are commonly reported sequelae of these methods of regional anesthesia. The problem of hypotension in the thoracic patient could be worsened if it is treated with fluid administration, especially after pneumonectomy. In contrast to the mechanism of action of TEA, ICN provides analgesia by somatosensory and motor blockade on the chest wall.[17]
The complications associated with intercostal blockade are due to systemic toxicity of local anesthetic drug[18] related to the large volume used. In our study, dilute concentrations of local anesthetic (0.2% bupivacaine) was used, and the total dose was kept below the maximum allowable dose (20 ml) to avoid the risk of systemic toxicity. Moreover, since the injections were done by the surgeon under direct vision, the danger of intravascular injection and also the injection into the spinal space were avoided. A rare complication of Harlequin syndrome associated with multilevel ICN has been reported.[19]
The most feared side complication of intrathecal and epidural opioids is respiratory depression.[20] The incidence of respiratory depression requiring intervention following conventional doses of intrathecal and epidural opioids is approximately 1%, which is the same as that following conventional dosing of intramuscular and intravenous opioids. In our study, none of the patients in either group had respiratory depression, which could be attributed to the use of epidural fentanyl in Group A which causes less respiratory depression than epidural morphine and the use of low dose of intravenous morphine in Group B.
Although there was no incidence of respiratory depression in our Group B patients, intravenous tramadol could be a suitable alternative for continuous infusion of morphine used in association with intercostal blockade, with the theoretical advantage of a lower risk of respiratory depression.[21]
The most common side effect of epidural opioids is pruritis[22] and the incidence is related to the type of opioids used, with epidural morphine being implicated more frequently than fentanyl or hydromorphone.[23] In this study, we used low dose of fentanyl 2 μ/cc in epidural infusion. The group of ICNs in this study was still prone to opioid-induced complications because morphine-based continuous intravenous analgesia additionally was used. Even though statistically insignificant, morphine-induced vomiting was more in Group B which could easily be managed with antiemetics.
Some of the absolute contraindications to epidural insertion do not necessarily exclude the use of a ICB. The safety placement of continuous intercostal nerve blockade even in a coagulopathic patient has been reported.[24] Difficult thoracic spinal anatomy makes an epidural technique more difficult and more likely to be associated with complications. In this situation, ICB technique can be performed under direct vision by the surgeon before the end of the surgical procedure. ICB has the added advantage in the setting of inadequate/failed epidural or if no epidural has been done preoperatively. Local or systemic sepsis and allergy to local anesthetic drugs contraindicate both an epidural block and ICB. In either of the groups, none of the patients had postoperative pulmonary infections or need for ventilator support/prolonged intensive care unit (ICU) stay or readmission. In either of the groups, none of the patients had postoperative pulmonary infections or need for ventilator support/prolonged ICU stay or readmission.
Limitations
In our study, the results failed to demonstrate a clear advantage of one group over the other in all aspects. No blinding was possible. We were not able to measure spirometry during the 1st postoperative day due to technical reasons. Shoulder pain which has been reported in over 75% of thoracotomy patients[25] has not been looked for in this study. Moreover, our patients were not followed up until their discharge, and no data were collected regarding the patient satisfaction regarding the quality of analgesia.
Conclusion
To summarize, even though the results showed a slightly better quality of analgesia with the thoracic epidural infusion of 0.1% bupivacaine and fentanyl for pain management after thoracotomy when compared with intercostal blockade, this difference is not clinically significant and a 5-segment intercostal blockade with bupivacaine and continuous intravenous infusion with morphine could be considered as a valid alternative for most patients undergoing thoracic surgery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mesbah A, Yeung J, Gao F. Pain after thoracotomy. BJA Educ. 2016;16:1–7. [Google Scholar]
- 2.Gottschalk A, Cohen SP, Yang S, Ochroch EA. Preventing and treating pain after thoracic surgery. Anesthesiology. 2006;104:594–600. doi: 10.1097/00000542-200603000-00027. [DOI] [PubMed] [Google Scholar]
- 3.Katz J, Jackson M, Kavanagh BP, Sandler AN. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain. 1996;12:50–5. doi: 10.1097/00002508-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Richardson J, Smith T, Tsiamis A, Shah RD. Postthoracotomy pain. Ann Thorac Surg. 1998;65:300–2. doi: 10.1016/s0003-4975(97)01117-x. [DOI] [PubMed] [Google Scholar]
- 5.Wheatley RG, Schug SA, Watson D. Safety and efficacy of postoperative epidural analgesia. Br J Anaesth. 2001;87:47–61. doi: 10.1093/bja/87.1.47. [DOI] [PubMed] [Google Scholar]
- 6.Joshi GP, Bonnet F, Shah R, Wilkinson RC, Camu F, Fischer B, et al. Asystematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg. 2008;107:1026–40. doi: 10.1213/01.ane.0000333274.63501.ff. [DOI] [PubMed] [Google Scholar]
- 7.Concha M, Dagnino J, Cariaga M, Aguilera J, Aparicio R, Guerrero M. Analgesia after thoracotomy: Epidural fentanyl/bupivacaine compared with intercostal nerve block plus intravenous morphine. J Cardiothorac Vasc Anesth. 2004;18:322–6. doi: 10.1053/j.jvca.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Perttunen K, Nilsson E, Heinonen J, Hirvisalo EL, Salo JA, Kalso E. Extradural, paravertebral and intercostal nerve blocks for post-thoracotomy pain. Br J Anaesth. 1995;75:541–7. doi: 10.1093/bja/75.5.541. [DOI] [PubMed] [Google Scholar]
- 9.Perttunen K, Kalso E, Heinonen J, Salo J. IV diclofenac in post-thoracotomy pain. Br J Anaesth. 1992;68:474–80. doi: 10.1093/bja/68.5.474. [DOI] [PubMed] [Google Scholar]
- 10.Power I, Bowler GM, Pugh GC, Chambers WA. Ketorolac as a component of balanced analgesia after thoracotomy. Br J Anaesth. 1994;72:224–6. doi: 10.1093/bja/72.2.224. [DOI] [PubMed] [Google Scholar]
- 11.Khalil KG, Boutrous ML, Irani AD, Miller CC, 3rd, Pawelek TR, Estrera AL, et al. Operative intercostal nerve blocks with long-acting bupivacaine liposome for pain control after thoracotomy. Ann Thorac Surg. 2015;100:2013–8. doi: 10.1016/j.athoracsur.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Rice DC, Cata JP, Mena GE, Rodriguez-Restrepo A, Correa AM, Mehran RJ. Posterior intercostal nerve block with liposomal bupivacaine: An alternative to thoracic epidural analgesia. Ann Thorac Surg. 2015;99:1953–60. doi: 10.1016/j.athoracsur.2015.02.074. [DOI] [PubMed] [Google Scholar]
- 13.Kristek J, Kvolik S, Sakić K, Has B, Prlić L. Intercostal catheter analgesia is more efficient vs. intercostal nerve blockade for post-thoracotomy pain relief. Coll Antropol. 2007;31:561–6. [PubMed] [Google Scholar]
- 14.Paqueron X, Lumbroso A, Mergoni P, Aubrun F, Langeron O, Coriat P, et al. Is morphine-induced sedation synonymous with analgesia during intravenous morphine titration? Br J Anaesth. 2002;89:697–701. [PubMed] [Google Scholar]
- 15.Lentschener C, Tostivint P, White PF, Gentili ME, Ozier Y. Opioid-induced sedation in the postanesthesia care unit does not insure adequate pain relief: A case-control study. Anesth Analg. 2007;105:1143–7. doi: 10.1213/01.ane.0000281441.93304.e3. [DOI] [PubMed] [Google Scholar]
- 16.Elsayed H, McKevith J, McShane J, Scawn N. Thoracic epidural or paravertebral catheter for analgesia after lung resection: Is the outcome different? J Cardiothorac Vasc Anesth. 2012;26:78–82. doi: 10.1053/j.jvca.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Sabanathan S, Smith PJ, Pradhan GN, Hashimi H, Eng JB, Mearns AJ. Continuous intercostal nerve block for pain relief after thoracotomy. Ann Thorac Surg. 1988;46:425–6. doi: 10.1016/s0003-4975(10)64657-7. [DOI] [PubMed] [Google Scholar]
- 18.Johnson MD, Mickler T, Arthur GR, Rosenburg S, Wilson R. Bupivacaine with and without epinephrine for intercostal nerve block. J Cardiothorac Anesth. 1990;4:200–3. doi: 10.1016/0888-6296(90)90238-b. [DOI] [PubMed] [Google Scholar]
- 19.Viswanath O, Wilson J, Hasty F. Harlequin syndrome associated with multilevel intercostal nerve block. Anesthesiology. 2016;125:1045. doi: 10.1097/ALN.0000000000001208. [DOI] [PubMed] [Google Scholar]
- 20.Bray RJ, Woodhams AM, Vallis CJ, Kelly PJ. Morphine consumption and respiratory depression in children receiving postoperative analgesia from continuous morphine infusion or patient controlled analgesia. Paediatr Anaesth. 1996;6:129–34. doi: 10.1111/j.1460-9592.1996.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 21.James MF, Heijke SA, Gordon PC. Intravenous tramadol versus epidural morphine for postthoracotomy pain relief: A placebo-controlled double-blind trial. Anesth Analg. 1996;83:87–91. doi: 10.1097/00000539-199607000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Cousins MJ, Mather LE. Intrathecal and epidural administration of opioids. Anesthesiology. 1984;61:276–310. [PubMed] [Google Scholar]
- 23.Goodarzi M. Comparison of epidural morphine, hydromorphone and fentanyl for postoperative pain control in children undergoing orthopaedic surgery. Paediatr Anaesth. 1999;9:419–22. doi: 10.1046/j.1460-9592.1999.00370.x. [DOI] [PubMed] [Google Scholar]
- 24.Ahn Y, Görlinger K, Alam HB, Eikermann M. Pain-associated respiratory failure in chest trauma. Anesthesiology. 2013;118:701–8. doi: 10.1097/ALN.0b013e318283996b. [DOI] [PubMed] [Google Scholar]
- 25.Gerner P. Postthoracotomy pain management problems. Anesthesiol Clin. 2008;26:355–67, vii. doi: 10.1016/j.anclin.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]