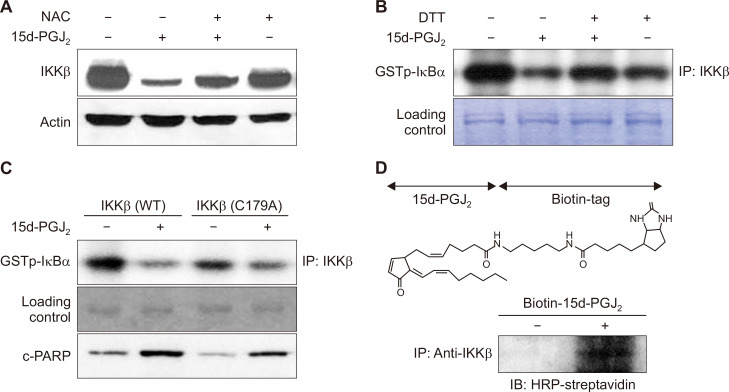
Figure 4. Downregulation of IKKβ is associated with induction of apoptosis in MCF10A-ras cells treated with 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2).
(A, B) MCF10A-ras cells were cotreated with N-acetyl-L-cysteine (NAC) (5 mM) or a thiol reducing agent, dithiothreitol (DTT) (500 μM), in the presence of 15d-PGJ2 (10 μM) for 24 hours. The expression level of IKKβ was determined by Western blot analysis. The proteins were immunoprecipitated by anti-IKKβ and subsequently incubated with glutathione S-transferase-IκB-a and [γ-32P]ATP for the kinase assay. Murine immunoglobulin G heavy chain band was used to ensure the equal lane loading. (C) MCF10A-ras cells were transfected with IKKβ and its mutant construct in which Cys179 is replaced by alanine. The catalytic activity of IKKβ and proteolytic cleavage of caspase-3 were determined by the kinase assay and Western blot analysis, respectively. (D) MCF10A-ras cells were treated with biotinylated 15d-PGJ2 (10 μM) for 12 hours. The biotinylated 15d-PGJ2-IKKβ complex was detected by the immunoprecipitation with IKKβ followed by Western blot analysis against horseradish peroxidase (HRP)-streptavidin as described in Materials and Methods.