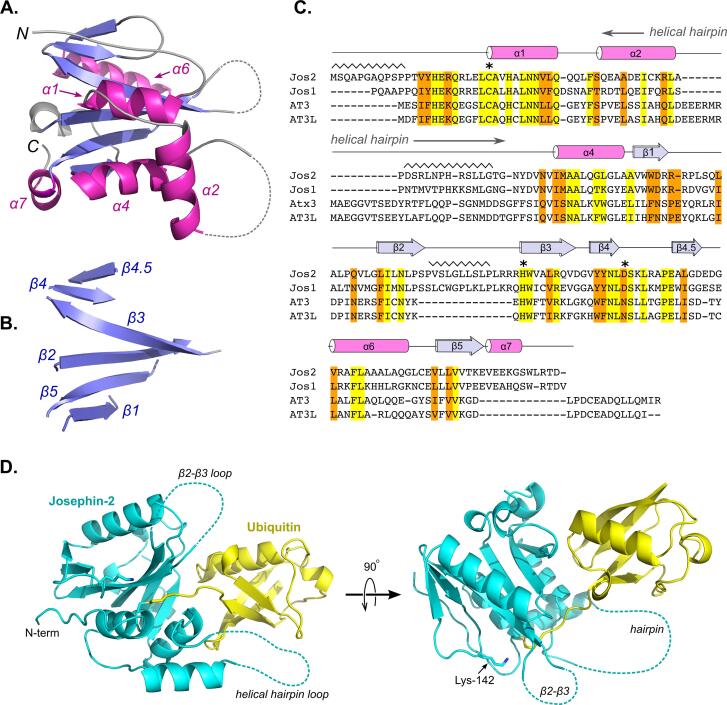
Fig. 1.
Human Josephin-2 adopts a compact α/β/α sandwich fold. (A) Cartoon representation of the Josephin-2 structure, with helices colored magenta and strands colored blue. A stereo version of this panel can be found in Fig. S1. (B) The same view as in panel A, but with the helices removed to reveal the central sheet. (C) Sequence alignment of the four members of the human MJD family. Abbreviations used: Jos2, Josephin-2; Jos1, Josephin-1, AT3, ataxin-3. Identities are colored yellow, and similar residues are colored orange. The secondary structure breakdown for Josephin-2 is shown above the sequence. Regions with the sawtooth symbol represent disordered stretches in the crystal structure, and the catalytic triad is marked by asterisks. The position of the helical hairpin in ataxin-3 and AT3L is also indicated. The alignment was prepared using TM-align and EMBOSS-Needle (Madeira et al., 2019, Zhang and Skolnick, 2005). (D) Two views of the complex between Josephin-2 (cyan) and ubiquitin (yellow). Large disordered loops in Josephin-2 are shown as dashed lines. The position of Lys-142 in the β4-β4.5 loop is shown in the right-hand panel. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)