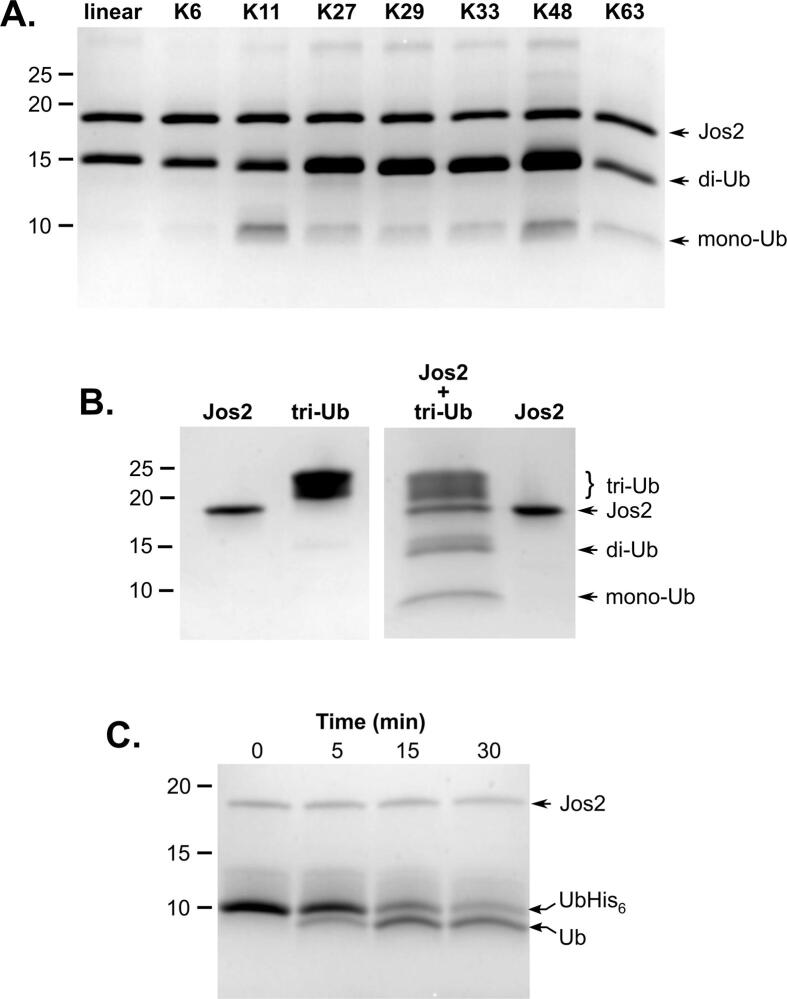
Fig. 4.
Activity of human Josephin-2 against different ubiquitin linkage types. (A) Activity of Josephin-2 versus a panel of ubiquitin dimers containing all naturally-occurring linkages. (B) Josephin-2 is able to cleave a branched K11/K48 tri-ubiquitin chain. Note that the ubiquitin trimer runs as a smeared band in this gel system. Panels A & B both show the results of a 20-hour incubation at 37°. (C) Josephin-2 cleaves the small Ub-His6 substrate more efficiently than ubiquitin dimers. A representative time course is shown for cleavage at room temperature.