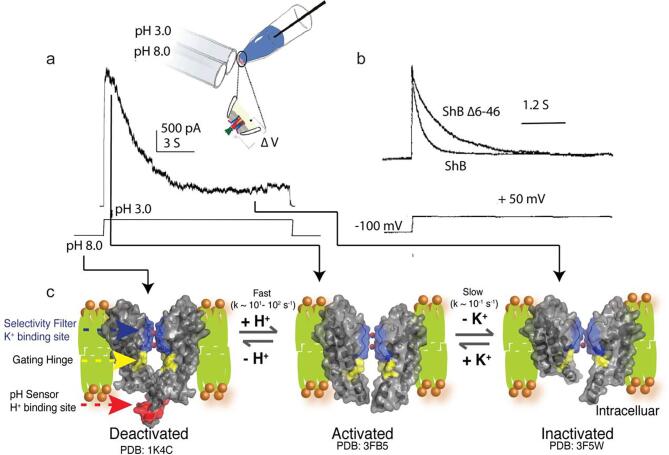
Fig. 3.
Inactivation of potassium channels. (a) The macroscopic current of KcsA in the patch-clamp measurement. KcsA is a pH-gated potassium channel. After activation by a pH drop from pH 8.0 to 3.0, the channel inactivates spontaneously and the current decreases gradually (b) The macroscopic current of Shaker measured by the patch-clamp technique. Shaker is a voltage-gated potassium channel, activated by switching the voltage from −100 to 50 mV. Shaker has two inactivation processes, the fast-decay current for the N-type inactivation and the slow-decay current for the C-type inactivation after the residues 6–46 were deleted. Adapted from reference (Sudha Hoshi et al., 1991). (c) Activation-coupled inactivation mechanism shown in KcsA. At pH 8.0, the channel is in the deactivated state; the activation gate is closed and the selectivity filter is in the conductive conformation. After the pH is dropped to 3.0, the channel converts to the activated state and both the activation gate and the selectivity filter are in the open conformation; potassium ions flow through the channel. The opening of the pH gate is also slowly coupled to the selectivity filter and biases the collapsed conformation. Therefore, the current decreases gradually until it reaches a plateau with minimal conductance.