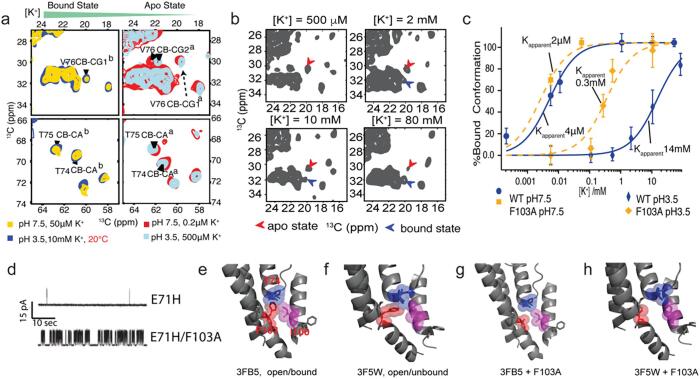
Fig. 4.
NMR chemical shifts indicate conformational changes and a potassium affinity change at the selectivity filter. (a) Similar chemical shift changes imply similar structural transitions for the selectivity filter of KcsA, regardless of pH, as indicated by the 13C–13C marker peaks of residues at the selectivity filter (e.g., T74, T75, V76). Presumably these structural changes correspond to conformational change between the conductive and collapsed selective filter seen in crystal structures. (b) Population of the K+-apo (collapsed) and K+-bound (conductive) states in the structural transitions modulated by ambient [K+]. (c) Titration experiments show that the potassium affinity changes by more than three orders of magnitude from 4 ± 1 μM for pH 7.5 and 14 ± 1 mM at pH 3.5. This indicates that the pH gate and the selectivity filter is strongly coupled at WT-KcsA. While similar measurements on the mutant F103A, in which C-type inactivation is greatly suppressed, a much smaller affinity change is measured, indicating a reduced coupling network. (d) Single channel recording experiments show that F103A can rescue the enhanced C-type inactivation caused by E71H, thus F103A reduces inactivation. (e–h) Structural models demonstrating the reduced coupling in F103A. The smaller side chain of alanine reduces the steric contact with neighbor residues such as T74 and I100. Adapted from the literature (Cuello et al., 2010b, Xu et al., 2017).