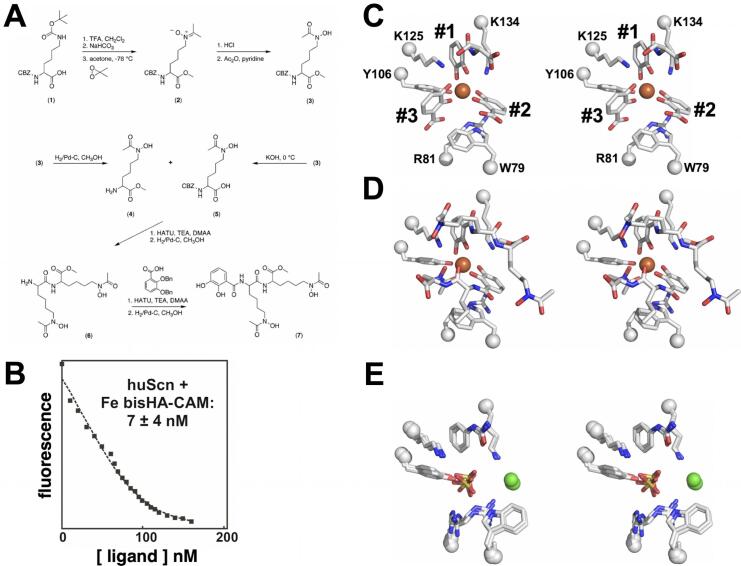
Fig. 3.
Identification of the Scn calyx pocket key for ligand recognition. (A) The stepwise synthesis of bisHA-CAM is detailed. (B) The binding of Fe-bisHA-CAM was quantitated by FQ as in (Abergel et al., 2006a, Abergel et al., 2006b, Goetz et al., 2002, Hoette et al., 2008, Miethke and Skerra, 2010): KD = 7 ± 4 nM. (C) For reference with subsequent structures, a stereoview of the binding of degraded Fe-ENT in the Scn calyx is detailed (compare with the modeled complex with intact Fe-ENT, Fig. 2B). The side-chains of key ligand-contacting residues and visualized ligand substituents are shown in a licorice-stick representation, colored by atom type (C, grey; N, blue; O, red; S, yellow; Fe, orange; Cl, green) and labeled, with Cα positions marked with spheres. Calyx pockets are numbered as in Fig. 1B. In this view, a DHBA-serine substituent occupies Pocket #1, and DHBA substituents occupy Pockets #2 and #3 (the DHBA group in Pocket #3 sits in an inverted orientation, carboxylate towards the protein, allowed by partial degradation of bound ENT). (D) The 1:2 Fe:bisHA-CAM complex is shown bound in the Scn calyx in the most-ordered molecule in the AU, in the same orientation and style as Fig. 3C. Two complete bisHA-CAM moieties are fully resolved and modeled. bisHA-CAM ligands are progressively less well ordered in the other two complexes in the crystal structure AU, but otherwise showed identical binding. (E) The superposition of all three molecules in the AU of the Scn/phenylurea complex structure (3TZS.pdb) are shown, in the same orientation and style as Fig. 3C, with the phenylurea ligands bound in Pocket #1. Note the centrally-bound sulfate ions and peripherally-bound chloride ions. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)