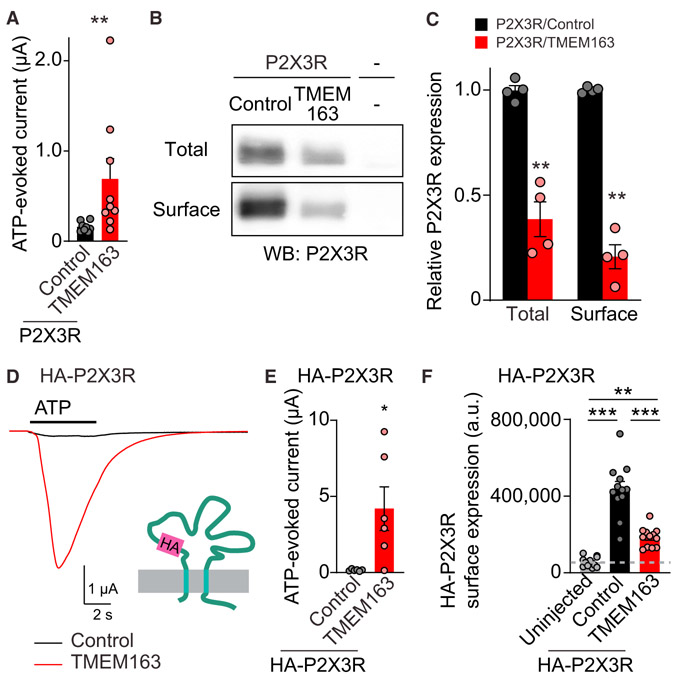
Figure 4. TMEM163 Enhances ATP-Evoked P2XR Channel Activity.
(A–C) Surface expression of P2X3R was biochemically measured in oocytes injected with cRNAs of 100 pg of P2X3R and 2 ng of TMEM163 or Neto2 (control). (A) TEVC recording (Vh = −30 mV, n = 8–9) showed an increase in 300 nM ATP-evoked currents by TMEM163 co-expression. (B) Total and surface expression of P2X3R in the oocytes. Proteins at the oocyte surface were biotinylated. The “Surface” and “Total” proteins were isolated with neutravidin and anti P2X3R antibody, respectively. (C) Quantification of P2X3R expression. P2X3R was detected in oocytes only injected with P2X3R cRNA, but not in “uninjected” oocytes, and co-injection of TMEM163 cRNA reduced both total and surface expression of P2X3Rs (n = 4).
(D–F) Agonist-evoked currents and chemiluminescently detected surface expression of extracellularly HA-tagged P2X3R (HA-P2X3R) were measured in cRNA-injected oocytes (cRNA amount injected: 1 ng of HA-P2X3; 2 ng of TMEM163 or Neto2 indicated as a control). (D and E) HA epitope was inserted into the extracellular domain of P2X3R (HA-P2X3R). TMEM163 robustly enhanced ATP (1 μM)-evoked currents of HA-P2X3R. Representative traces (D) and summary bar graph (E) are shown (n = 6). (F) Surface expression of HA-P2X3R in oocytes was measured chemiluminescently. HA proteins at the oocyte surface were detected with anti-HA antibody under non-permeabilized conditions as chemiluminescence signal (n = 10). The signal from uninjected oocytes indicates background in this assay.
Data are mean ± SEM. Mann-Whitney U-test (A and E), unpaired t test (C), one-way ANOVA with Bonferroni’s post-test (F); *p < 0.05; **p < 0.01; ***p < 0.001.