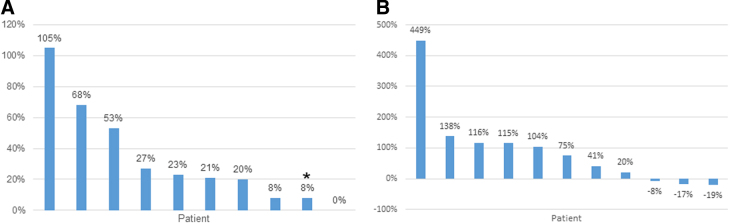
FIG. 2.
Tumor response. (A) The best overall response by RECIST v. 1.1. *New lesion. One patient had progression of disease but was not evaluable by RECIST v. 1.1, and one patient was not evaluable due to dose-limiting toxicity before tumor reassessment. (B) The best change in serum CA19-9. One patient had a tumor without positive CA19-9. RECIST, response evaluation criteria in solid tumors.