Figure 1. LPS in the Cytosol of Endothelial Cells Activates cGAS-STING Suppression of YAP1.
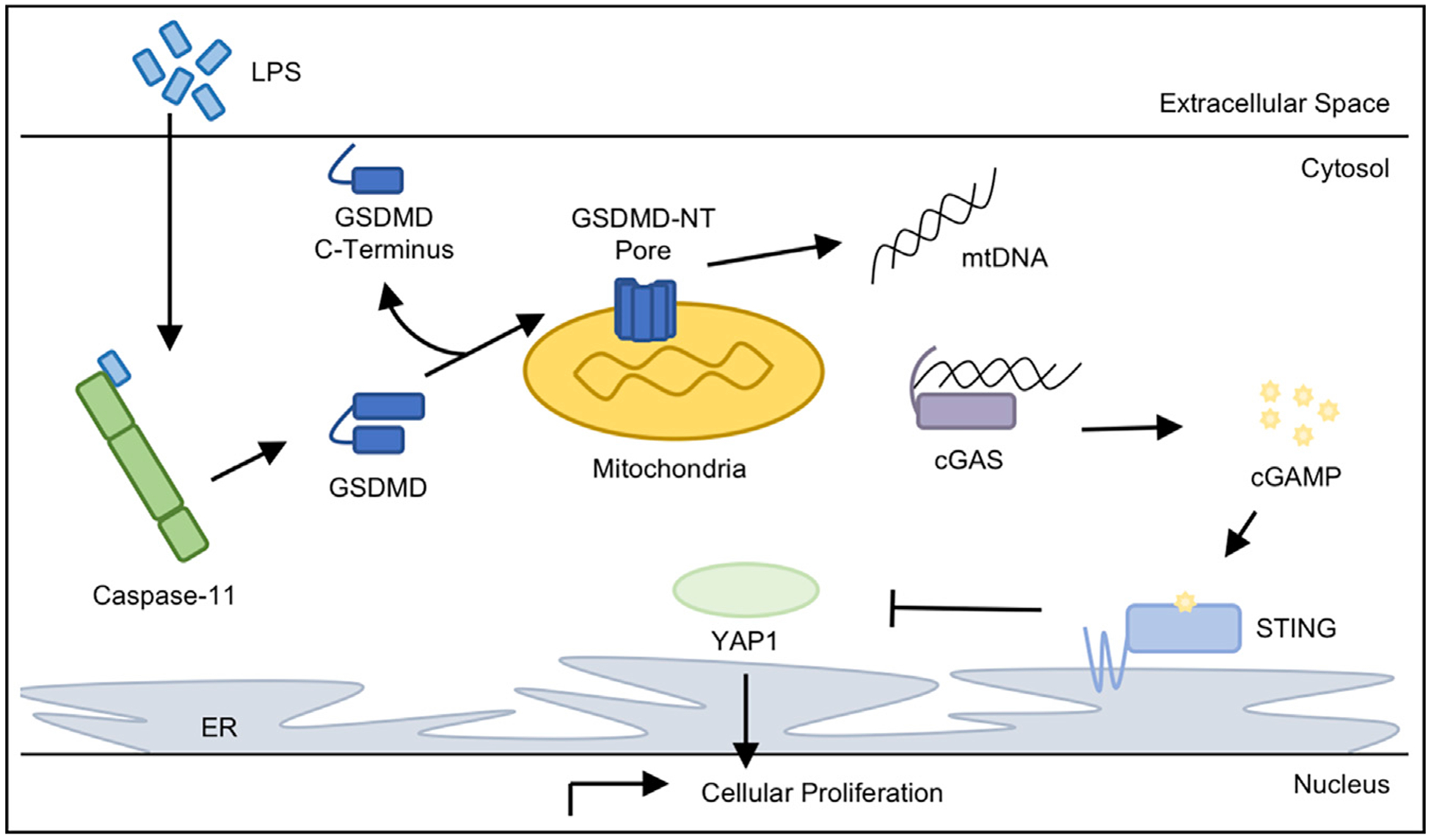
Intracellular LPS stimulates murine caspase-11 to form the noncanonical inflammasome and cleave GSDMD. Canonically, the cleaved GSDMD-NT forms a pore in the plasma membrane to cause cellular ion gradient depolarization and the release of inflammatory cytokines to facilitate pyroptotic cell death. However, Huang and colleagues discovered that LPS-stimulated cleavage of GSDMD in murine endothelial cells leads to the concentration of the GSDMD-NT on the mitochondria, presumably perforating the mitochondrial membrane. GSDMD-NT pore formation on the mitochondria releases mtDNA into the cytosol, where it is detected by the cytosolic DNA sensor cGAS. Upon binding mtDNA, cGAS produces the secondary messenger cGAMP, which is detected by the adaptor protein STING. Here, STING activation through cGAMP binding inhibited YAP1 translocation into the nucleus, preventing transcriptional upregulation of genes promoting cellular proliferation.
