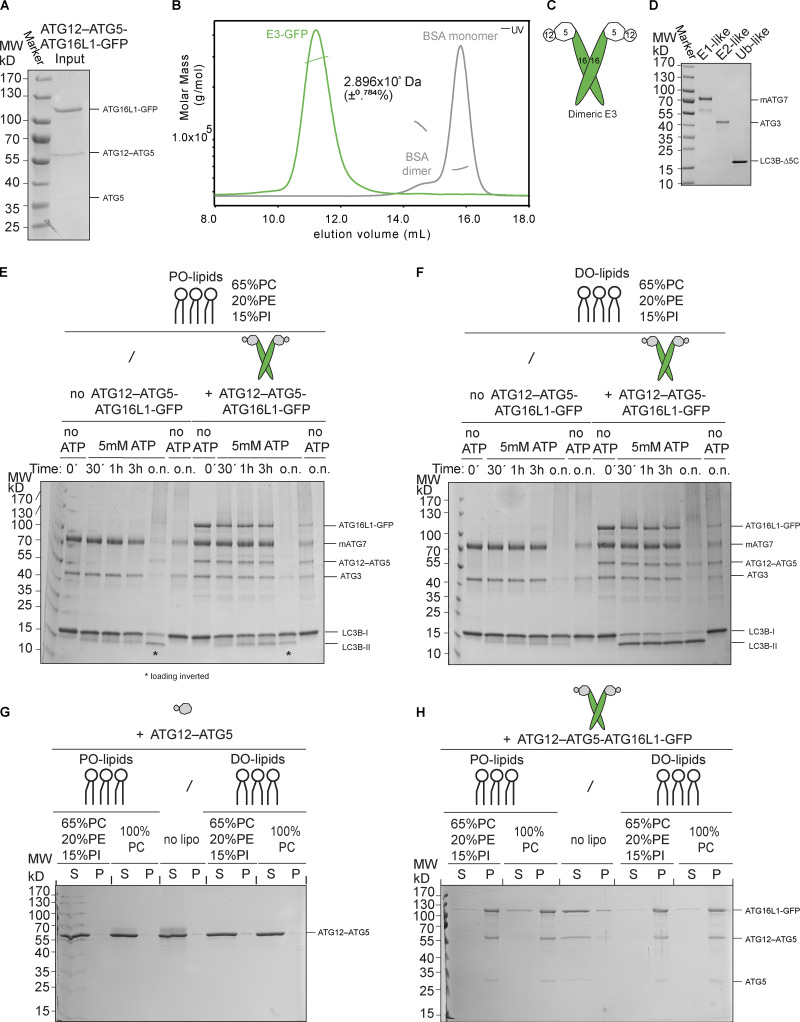
Figure S1.
Biochemical characterization of the E3 and the LC3 lipidation machinery. (A) Purified human E3-GFP complex resolved on a 10% SDS-polyacrylamide gel and stained with Coomassie Brilliant Blue. MW, molecular weight. (B) SLS plot of recombinant E3-GFP. The protein was applied onto a Superose 6 Increase 10/300 GL column coupled with a TREOS II instrument. BSA was used for calibration. (C) Schematic representation of the dimeric E3-like ligase holo-complex containing two copies of each subunit of ATG12, ATG5, and ATG16L1(+monoGFP). (D) Recombinant mouse ATG7, human ATG3, and LC3BΔ5C resolved on a 10% SDS-polyacrylamide gel and stained with Coomassie Brilliant Blue. MW, molecular weight. (E) In vitro LC3B lipidation assay using PO-SUVs (65% PC:15% liver PI:20% PE), ATG7, ATG3, E3-GFP (1 µM), and LC3B incubated at 37°C in the presence of MgCl2/ATP. Samples taken at the indicated time points (o.n.) were loaded on a 4–15% SDS-polyacrylamide gel. Time points corresponding to o.n. incubation indicated with an asterisk (*) were swapped during loading. MW, molecular weight. (F) ATG7, ATG3, E3-GFP (1 µM), and LC3B were incubated with DO-SUVs (65% PC:15% PI:20% PE) at 37°C with MgCl2 and ATP. Samples at the indicated time points (o.n.) were loaded on a 4–15% SDS-polyacrylamide gel. MW, molecular weight. (G) Co-sedimentation assay of ATG12–ATG5 with DO-lipid (left) or PO-lipid (right) SUVs (65% PC:15% PI: 20% PE). MW, molecular weight. (H) Co-sedimentation assay of the E3 with DO-lipid (left) or PO-lipid (right) SUVs with the indicated lipid composition. MW, molecular weight.