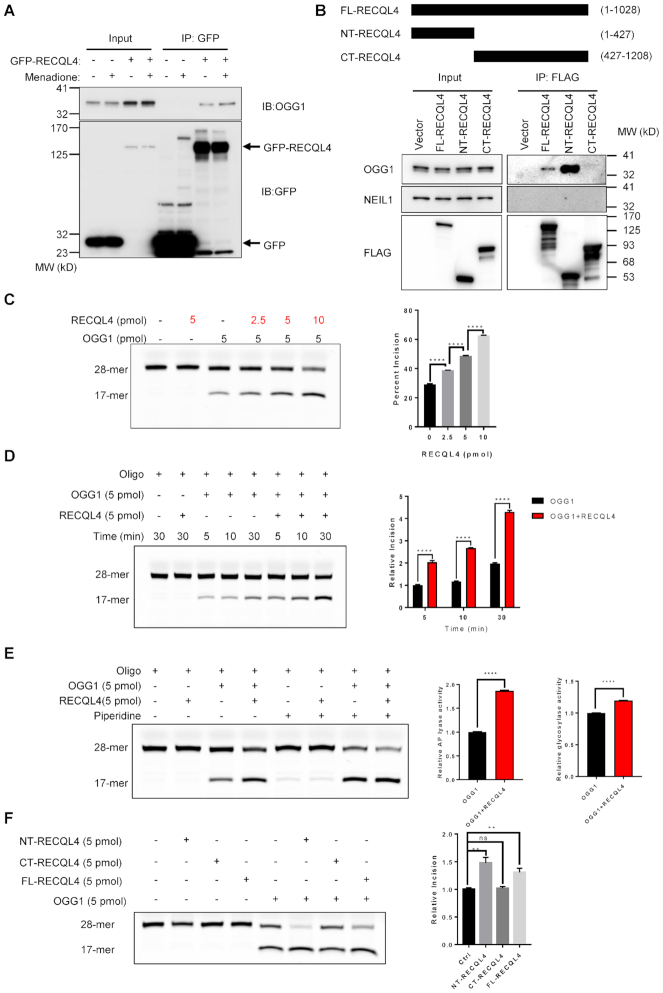
Figure 3.
RECQL4 selectively interacts with OGG1 and promotes 8-oxoG repair. (A) Menadione treatment enhances RECQL4/OGG1 interaction. Immunoprecipitated material from cells expressing GFP or GFP-tagged WT-RECQL4 with GFP-TRAP beads analyzed by immunoblotting (IB) with anti-GFP and anti-OGG1 antibodies. (B) N-terminal domain of RECQL4 interacts with OGG1 in cells. Schematic diagram of 3× FLAG-tagged RECQL4 and the truncated fragments (upper panel). FL-RECQL4, NT-RECQL4, and CT-RECQL4 were all tagged with both 3× FLAG and SV40 nuclear-location sequence (NLS). (C) Representative gel (left) and quantification (right) of the stimulation of catalytic activity of OGG1 by RECQL4 in a dose-dependent manner. Data are presented as mean ± SEM from three independent experiments. ****P < 0.0001, using unpaired two-tailed Student's t test. (D) Representative gel (left) and quantification (right) of the stimulation of catalytic activity of OGG1 by RECQL4 in a time-course dependent manner. Data are presented as mean ± SD from two independent experiments. ****P < 0.0001, using unpaired two-tailed Student's t test. (E) Representative gel (left) of the stimulation of catalytic activity of OGG1 by RECQL4 treated with or without piperidine. Quantification (right) of the glycosylase and AP lyase activities of OGG1 stimulated by RECQL4. Data are presented as mean ± SD from two independent experiments. ****P < 0.0001, using unpaired two-tailed Student's t test. (F) BER assay showing that the N-terminal domain of RECQL4 functionally interacts with OGG1. 3 × FLAG-tagged RECQL4 proteins were purified from normal U2OS cell. A representative image (left) and quantifications (right) are shown. **P < 0.01; ns, not significant, using unpaired two-tailed Student's t test. Data are shown as mean ± SEM, n = 4.