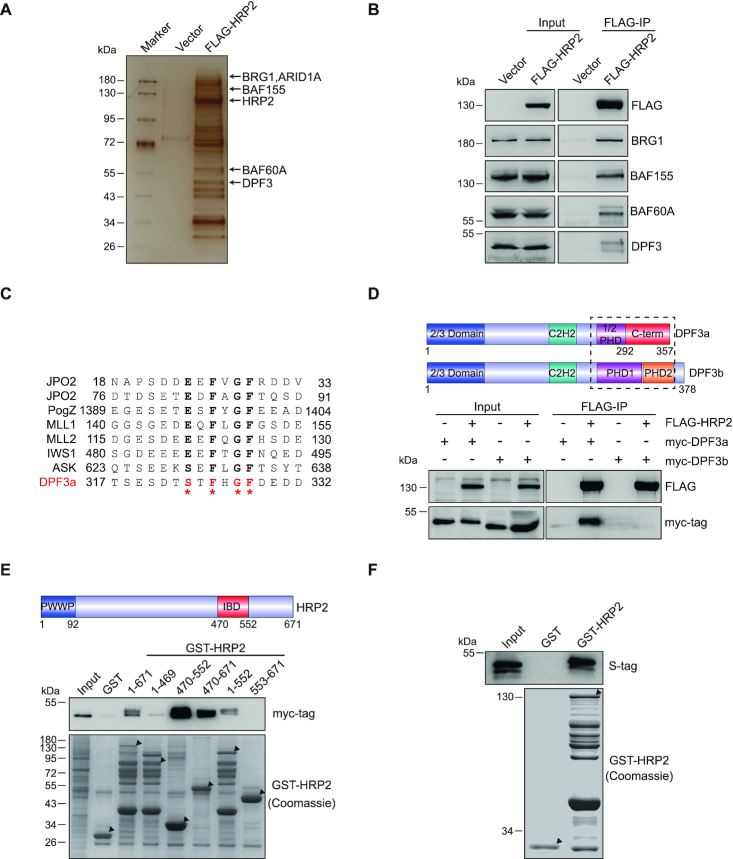
Figure 2.
HRP2 directly interacts with DPF3a. (A) 293T cells were transfected with FLAG-HRP2 and whole cell lysates were subjected to co-IP. Isolated HRP2 binding proteins were visualized by silver staining. (B) Co-IP analysis of the interactions between FLAG-HRP2 and BAF complex subunits in 293T cells. (C) Alignment of DPF3a with other IBD binding protein sequences shows that DPF3a possesses a typical IBD binding motif (IBM). (D) Schematic representation of the DPF3 isoforms. DPF3a contains a half PHD finger and a specific C-terminus, whereas DPF3b contains a double PHD finger. Blue: 2/3 domain; teal: C2H2-Krüppel-like zinc finger; purple: first plant homeodomain; orange: second plant homeodomain; red: DPF3a-specific C-terminus (upper). Co-IP analysis of the interaction between FLAG-HRP2 and myc-DPF3a or myc-DPF3b in 293T cells (lower). (E) HRP2 IBD domain is essential for DPF3a binding. Schematic representation of HRP2. Amino acid positions are depicted (upper). GST pull-down assays examining the interactions between GST-HRP2 (WT and its truncated mutants as indicated) and myc-DPF3a shows that the IBD domain of HRP2 is indispensable for DPF3a binding (lower). (F) S-tag-tagged DPF3a was purified in bacteria and subjected to GST pull-down assay together with GST-HRP2. The result shows that HRP2 interacts with DPF3a directly in vitro.