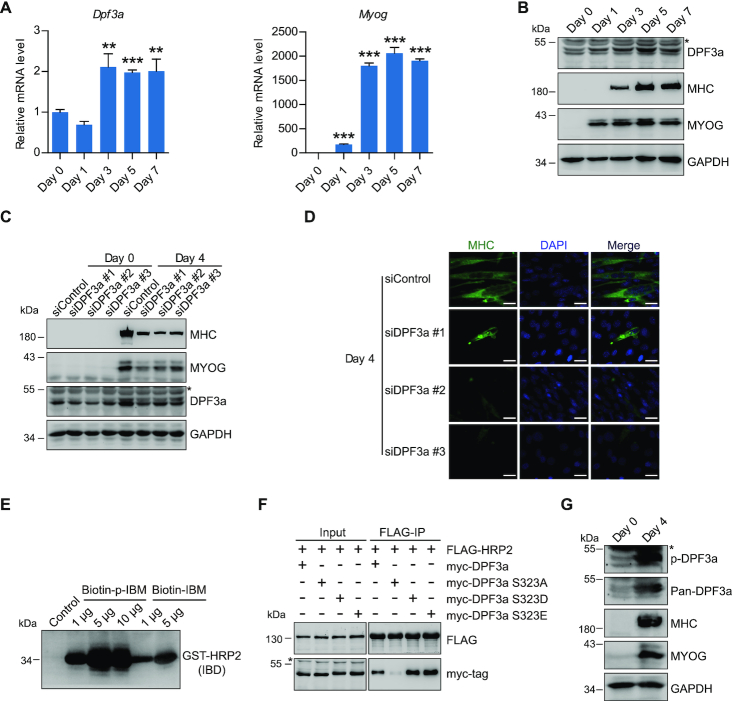
Figure 4.
DPF3a is critical for C2C12 differentiation, and elevation of DPF3a expression and phosphorylation in myotubes enhances its binding to HRP2. (A) RT–qPCR analysis for Dpf3a (left) and Myog (right) transcripts of myoblasts and myotubes at various differentiation stages as indicated. Data represent as means ± SD of three replicates. ** P < 0.01 and *** P < 0.001. (B) Western blotting analysis of DPF3a, MYOG, MHC and GAPDH in myoblasts and myotubes at various differentiation stages as indicated. (C) Western blotting analysis of MYOG, MHC, DPF3a and GAPDH in myoblasts and myotubes transfected with scramble and three DPF3a-specific siRNAs as indicated. (D) Immunofluorescence analysis of MHC and DAPI in myotubes transfected with scramble and three different DPF3a siRNAs. Scale bars, 25 μm. DAPI, 4′,6-diamidino-2-phenylindole. (E) Biotin-labeled DPF3a or p-DPF3a peptide was incubated with GST-IBD. Peptide pull-down assay shows that phosphorylation of DPF3a enhanced its affinity for HRP2. (F) Co-IP assay examining the interaction of FLAG-HRP2 and indicated myc-DPF3a (WT or mutants). (G) Western blotting analysis of DPF3a, p-DPF3a, MHC, MYOG and GAPDH in myoblasts and myotubes showing that phosphorylation of DPF3a increased upon cell differentiation.