Figure 1.
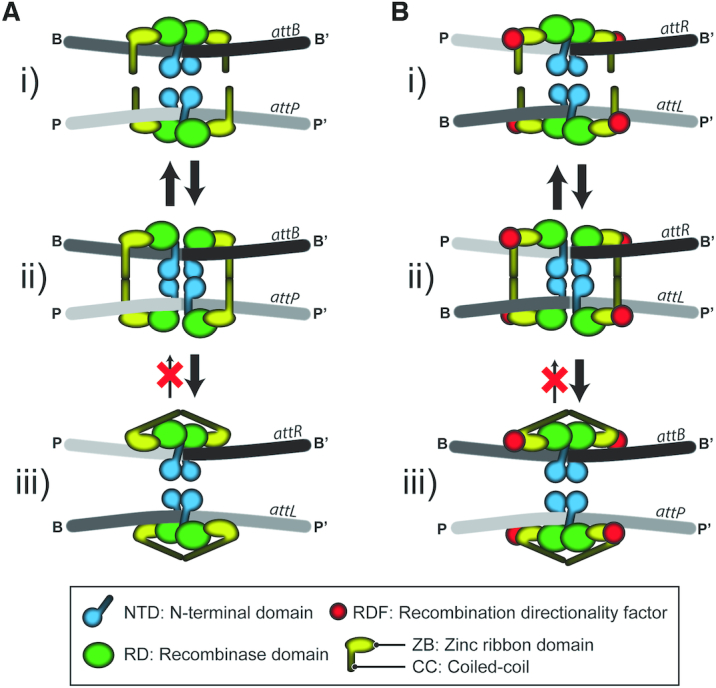
Phage integration (attP x attB → attL + attR) and excision (attL x attR → attP + attB) mediated by serine integrases; control of recombination directionality by RDF. The integration and excision reactions are schematically diagrammed in panels (A) and (B), respectively. The pre-chemical steps of the reaction involve binding of Int dimers to att sites (I) followed by synapsis of the bound sites (II). In the functional synapse, the sites are paired in a complementary fashion, that is, a P-type half-site is paired with a B-type half-site between the synaptic partners. The ensuing chemical/conformational steps include DNA cleavage, 180° rotation of the cleaved complex and relegation of the broken strands (III). The reaction is irreversible because of the auto-inhibited state of the post-recombination Int-att site complex. In the schematic representation of Int, the amino-terminal domain (NTD) is distinguished from the CTD comprised of a tripartite motif, namely, a recombinase domain (RD), a zinc ribbon domain (ZD) and a coiled coil (CC) region. RDF regulates the directionality of recombination by modulating the CC trajectories. The presence of RDF promotes the auto-inhibited state of Int bound attP-attB, while the absence of RDF has a similar effect on Int bound attL-attR. This figure follows the model for the recombination synapse proposed from the structure of the L1 integrase CTD bound to the A118 attP half-site (23).
