Figure 10.
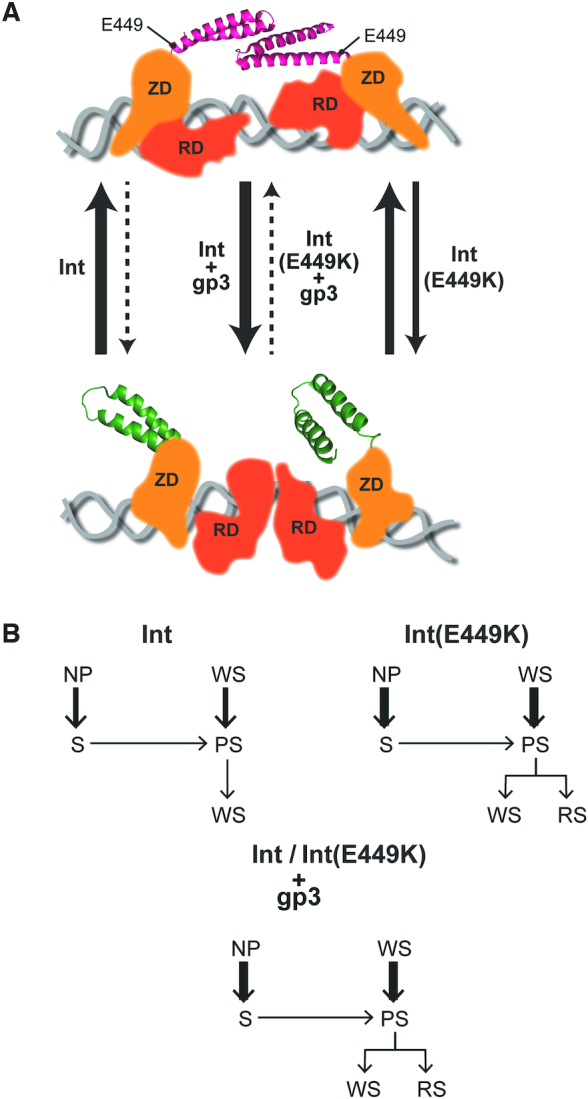
Comparison of E449K with gp3 in the activation of Int for attL × attR recombination. (A) The closed arrangement of the CC motifs (shown in red) within the Int dimer bound to attL (or attR), which proscribes functional synapsis, is schematically diagrammed at the top. RDF (gp3) shifts the equilibrium toward the open conformation of the coiled coils (shown in green), fostering inter-dimer interactions and productive synapsis (bottom schematic). E449K mimics gp3 in its thermodynamic effect; however, the magnitude of the effect is considerably lower for E449K. The representations follow the proposed structural model for the L1 integrase (23,28). The CC trajectories in the bottom and top panels correspond to the CC1 and CC2 designations, respectively, in the structure. The relative thickness of the arrows qualitatively indicates the direction of the equilibrium between synapsis-competent and synapsis-incompetent conformations of Int and Int(E449K) with and without gp3. As the ϕC31 Int structure has not been solved, the location of E449 shown here at the base of CC is only an approximation. RD = recombinase domain; ZD = zinc finger domain. (B) The kinetic re-routing of the WS and NP complexes toward synaptic complexes is schematically diagrammed. The thickness of the arrows representing k-WSd and k-NPd qualitatively conveys the speed of WS and NP dissociation, respectively, for Int and Int(E449K) with and without gp3.
