Table 1.
Alkene Scope of 1,2-Arylboration[a]
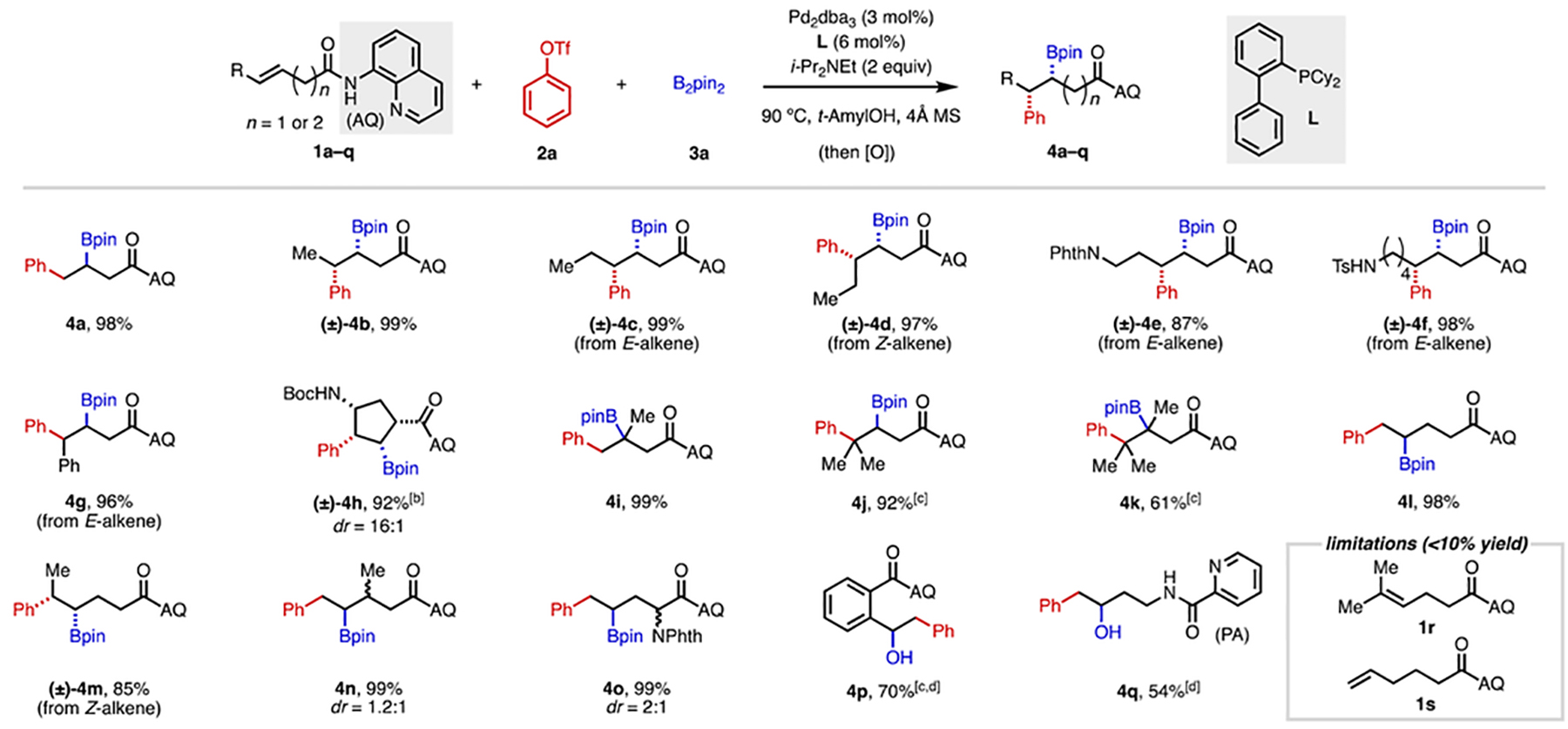
|
Reaction conditions: 1a–q (0.05 mmol or 0.1 mmol), 2a (1.5 equiv), 3a (2 equiv), Pd2dba3 (3 mol%), L (6 mol%), i-Pr2NEt (2 equiv), 4Å MS (15–30 mg), 90 °C, N2, 38–44 h. Percentages refer to isolated yields. Unless otherwise noted, diastereomeric ratio (dr) was found to be >30:1 in all cases. [b] 2a (3 equiv), 3a (3 equiv), Pd2dba3 (5 mol%), L (10 mol%), i-Pr2NEt (3 equiv), 100 °C. [c] 2a (2 equiv), 3a (3 equiv), Pd2dba3 (4.5 mol%), L (9 mol%), i-Pr2NEt (3 equiv). [d] The final product was oxidized to the corresponding alcohol with NaBO3•4H2O (5 equiv) for ease of isolation.
