Table 5.
Diastereoselective Arylboration Using a Removable Chiral Directing Group.[a]
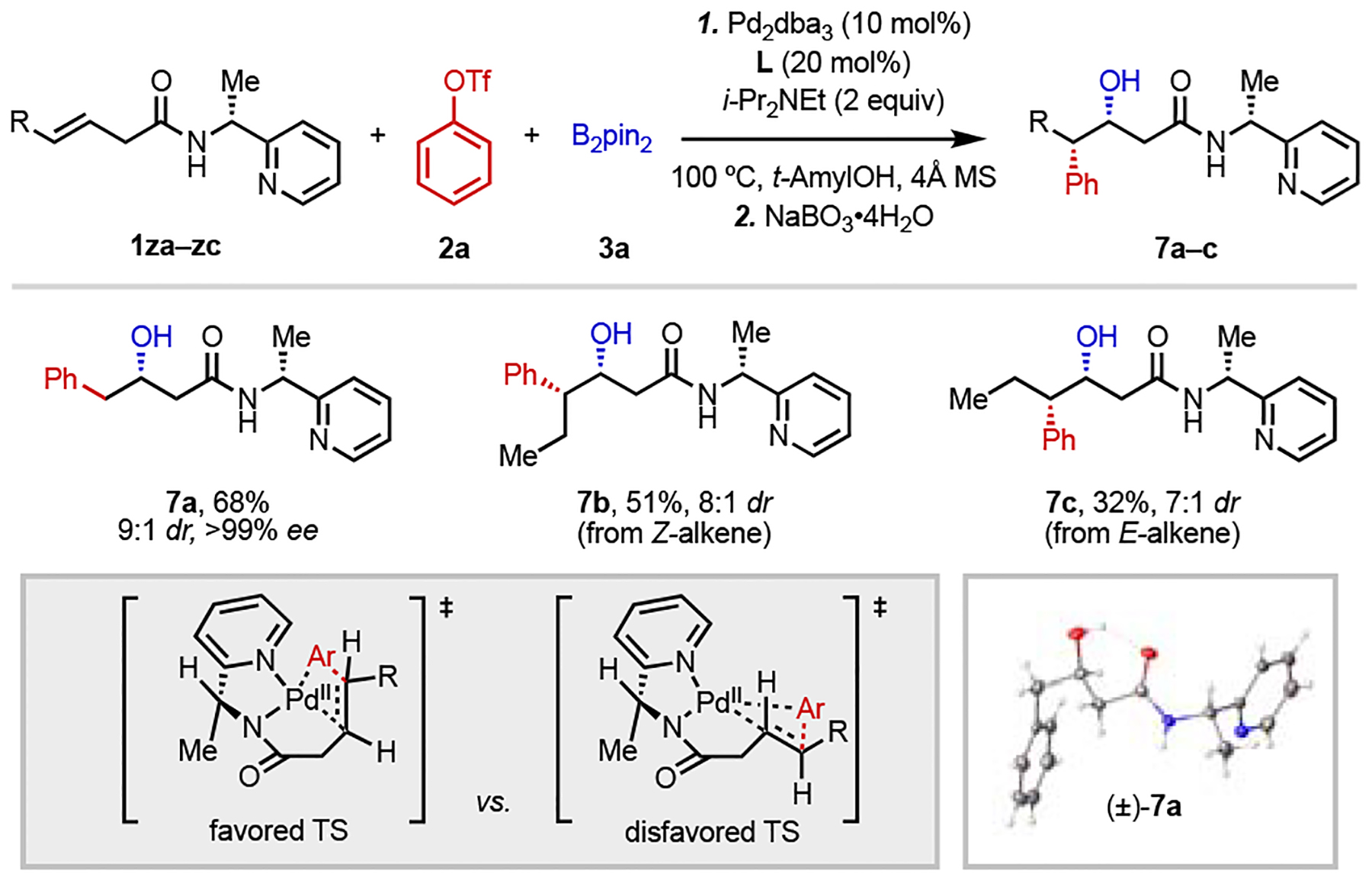
|
Reaction conditions: 1za–zc (0.05 mmol), 2a (1.5 equiv), 3a (2 equiv), Pd2dba3 (10 mol%), L (20 mol%), i-Pr2NEt (2 equiv), 4Å MS (20 mg), 100 °C, N2, 44–48 h. The final products were oxidized to the corresponding alcohol with NaBO3•4H2O (5 equiv) for ease of isolation. All the yields refer to the isolated yields.
